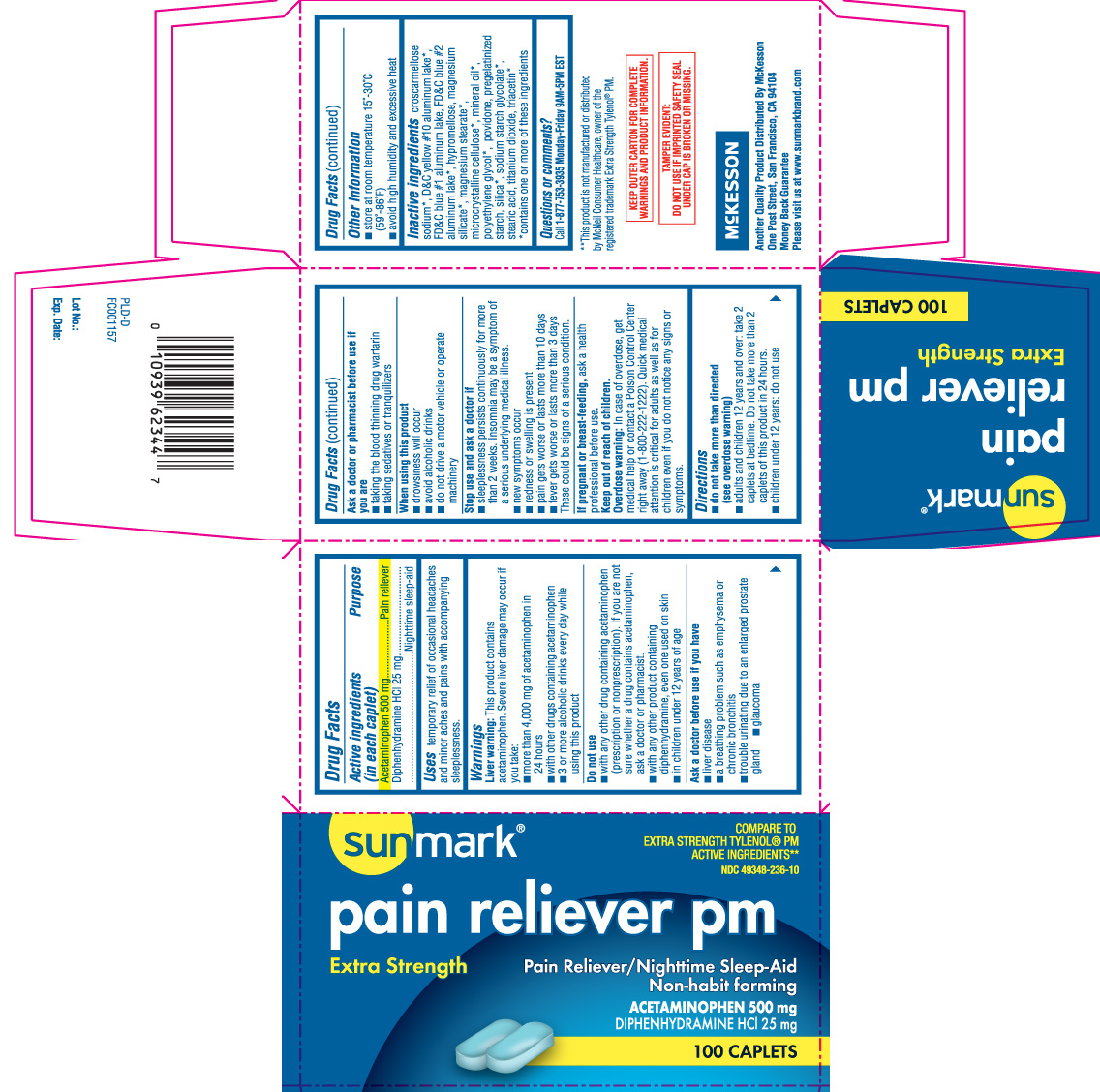
PAIN RELIEVER PM EXTRA STRENGTH
-
acetaminophen and
diphenhydramine hydrochloride tablet
McKesson (Sunmark)
Acetaminophen 500 mg
Diphenhydramine HCl 25 mg
Pain reliever
Nighttime sleep- aid
temporary relief of occasional headaches and minor aches and pains with accompanying sleeplessness.
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
These could be signs of a serious condition
ask a health professional before use.
Overdose warning: In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222). Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
| adults and children 12 years and over |
|
| children under 12 years |
|
croscarmellose sodium*, D and C Yellow #10 Aluminum Lake*, FD and C Blue #1 Aluminum Lake, FD and C Blue #2 Aluminum Lake*, hypromellose, magnesium silicate*, magnesium stearate*, microcrystalline cellulose*, mineral oil*, polyethylene glycol*, povidone, pregelatinized starch, silica*, sodium starch glycolate*, stearic acid, titanium dioxide, triacetin*
*contains one or more of these ingredients
Call 1-877-753-3935 Monday- Friday 9AM- 5PM EST
Compare to EXTRA STRENTH Tylenol® PM active ingredients **
Pain Reliever PM
Extra Strength
Pain reliever /nighttime Sleep-Aid
Non- habit forming
Acetaminophen 500 mg
Diphenhydramine HCl 25 mg
Tamper Evident: Do not use if imprinted safety seal under cap is broken or missing
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION
**This product is not manufactured or distributed by McNeil Consumer Healthcare, owner of the registered trademark Tylenol® PM.
Pain Reliever PM caplets
|
PAIN RELIEVER PM
EXTRA STRENGTH acetaminophen, diphenhydramine hcl tablet | ||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC MONOGRAPH NOT FINAL | part343 | 03/26/2013 | |
| Labeler - McKesson (Sunmark) (177667227) |
| Registrant - P and L Development of New York Corporation (800014821) |