ABILIFY MAINTENA KIT
-
aripiprazole injection, powder, lyophilized, for suspension, extended release
Otsuka America Pharmaceutical, Inc.
ARIPIPRAZOLE MAINTENA LabelingABILIFY MAINTENA KIT
Aripiprazole for extended release injectable suspension 300mg per vial
Label of kit for 300 mg per vials
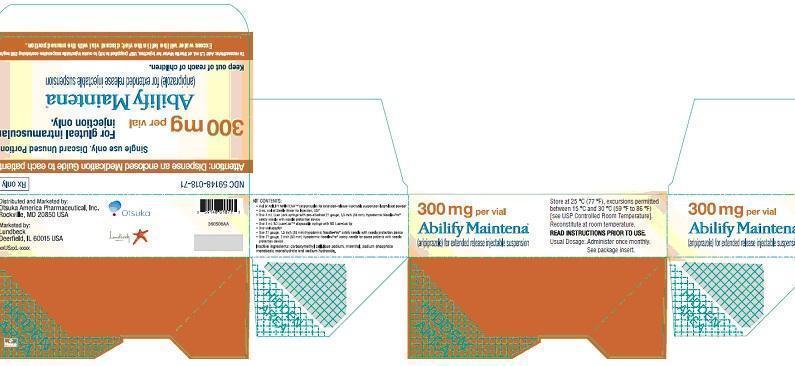
ARIPIPRAZOLE MAINTENA KIT
Aripiprazole for extended release injectable suspension 400mg per vial
Label of kit for 400 mg per vials

ABILIFY MAINTENA KIT
aripiprazole
injection, powder, lyophilized, for suspension, extended release
|
|
|
|
|
|
ABILIFY MAINTENA KIT
aripiprazole
injection, powder, lyophilized, for suspension, extended release
|
|
|
|
|
|
Revised: 04/2013Otsuka America Pharmaceutical, Inc.

