ALCOHOL PREP PAD
-
isopropyl alcohol swab
ZEE MEDICAL
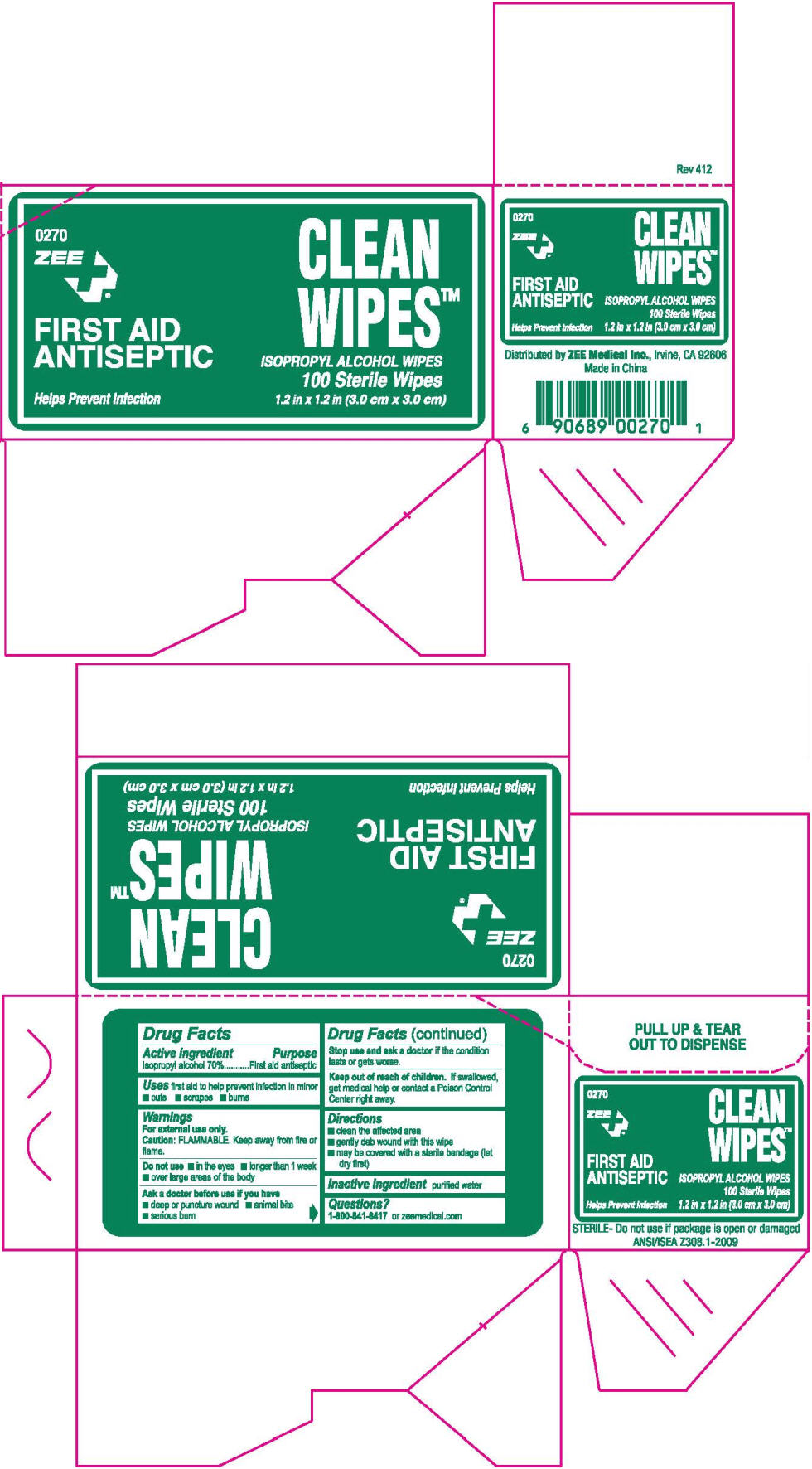
Drug Facts
Isopropyl alcohol 70%
First aid antiseptic
first aid to help prevent infection in minor
For external use only.
FLAMMABLE. Keep away from fire or flame.
Stop use and ask a doctor if the condition lasts or gets worse.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
purified water
1-800-841-8417 or zeemedical.com
Distributed byZEE Medical Inc., Irvine, CA 92606
0270
ZEE
FIRST AID
ANTISEPTIC
Helps Prevent Infection
CLEAN
WIPES™
ISOPROPYL ALCOHOL WIPES
100 Sterile Wipes
1.2 in x 1.2 in (3.0 cm x 3.0 cm)
|
ALCOHOL PREP PAD
isopropyl alcohol swab | ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC MONOGRAPH FINAL | part344 | 01/15/2013 | |
| Labeler - ZEE MEDICAL (009645623) |
| Registrant - DUKAL Corporation (791014871) |