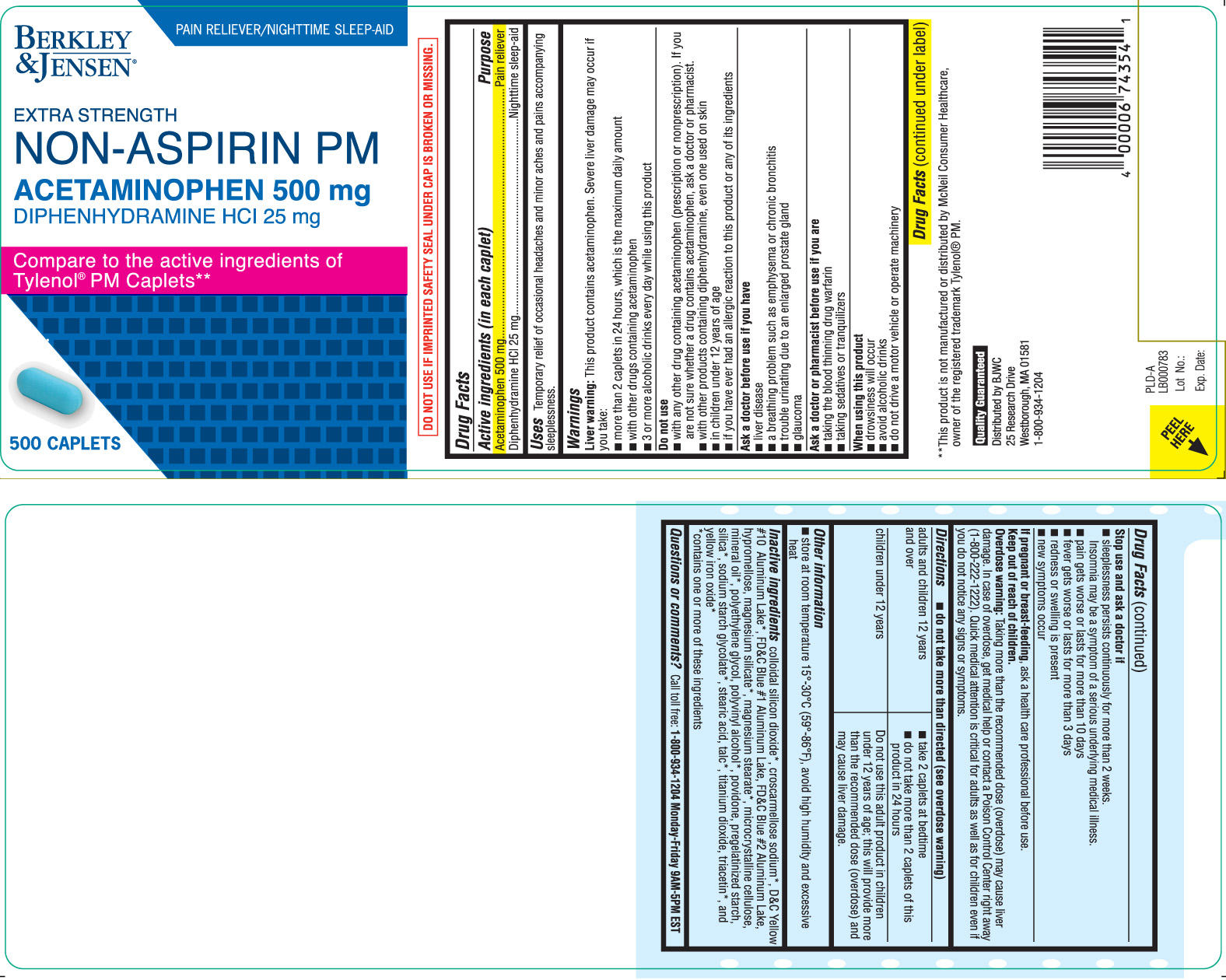
ACETAMINOPHEN PM EXTRA STRENGTH
-
acetaminophen and
diphenhydramine hydrochloride tablet, coated
BJWC (Berkley & Jensen / BJ's)
Acetaminophen 500 mg
Diphenhydramine HCl 25 mg
Pain reliever
Nighttime sleep-aid
Temporary relief of occasional headaches and minor aches and pains accompanying sleeplessness.
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
ask a health care professional before use.
Overdose warning: Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222). Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
| adults and children 12 years and over |
|
| children under 12 years | Do not use this adult product in children under 12 years of age, this will provide more than the recommended dose (overdose) and may cause liver damage. |
Call toll free: 1-800-934-1204 Monday-Friday 9AM-5PM EST
PAIN RELIEVER/NIGHTTIME SLEEP-AID
EXTRA STRENGTH
NON-ASPIRIN PM
ACETAMINOPHEN 500 mg
DIPHENHYDRAMINE HCl 25 mg
Compare to the active ingredients of Tylenol® PM Caplets**
CAPLETS
DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
**This product is not manufactured or distributed by McNeil Consumer Healthcare, owner of the registered trademark Tylenol® PM.
Distributed by BJWC
25 Research Drive
Westborough, MA 01581
1-800-934-1204
BERKLEY & JENSEN EXTRA STRENGTH NON-ASPIRIN PM CAPLETS
|
ACETAMINOPHEN PM
EXTRA STRENGTH acetaminophen, diphenhydramine hcl tablet, coated | ||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC MONOGRAPH NOT FINAL | part343 | 01/15/2013 | |
| Labeler - BJWC (Berkley & Jensen / BJ's) (159082692) |
| Registrant - P and L Development of New York Corporation (800014821) |