ACETAMINOPHEN, DEXTROMETHORPHAN HYDROBROMIDE, GUAIFENESIN, PHENYLEPHRINE HYDROCHLORIDE
-
acetaminophen,
dextromethorphan hydrobromide,
guaifenesin and
phenylephrine hydrochloride tablet, coated
AAA Pharmaceutical, Inc.
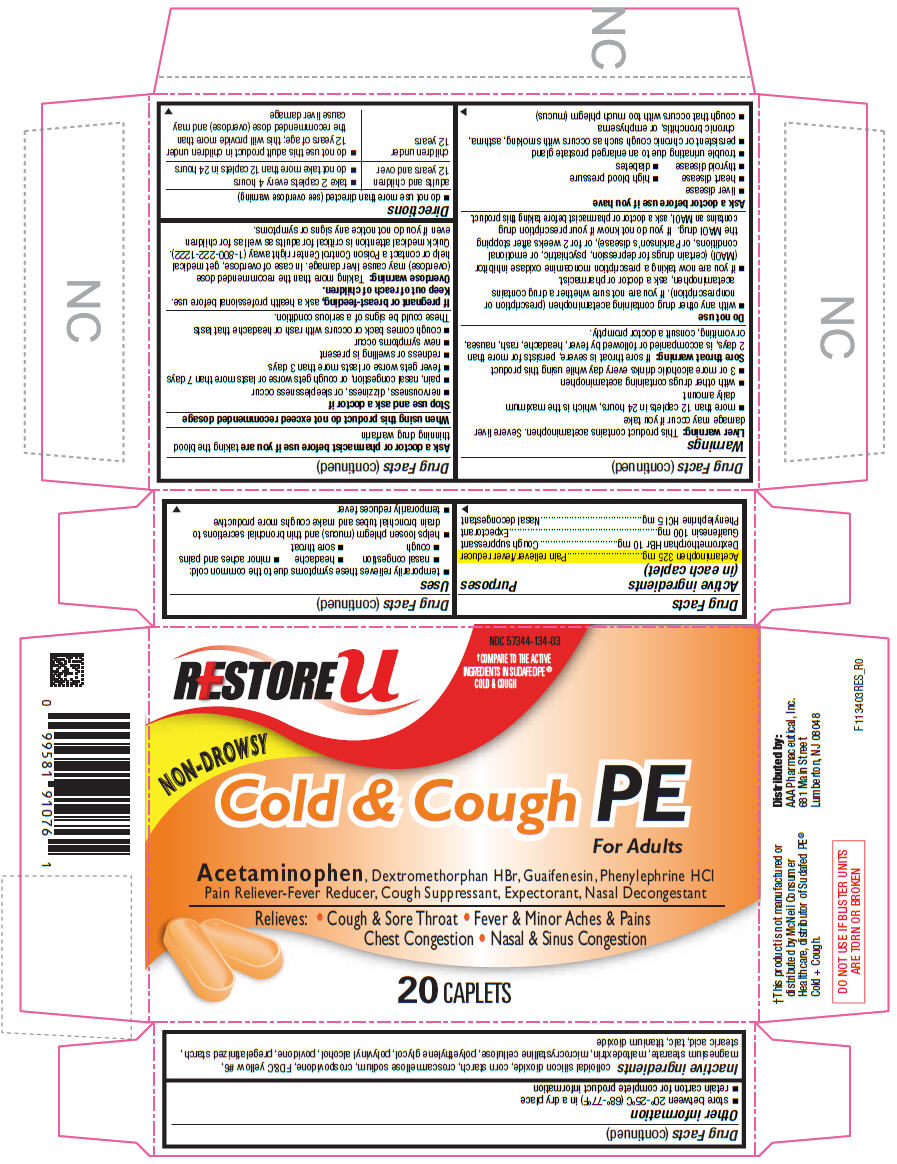
Drug Facts
| Active ingredients (in each caplet) |
Purposes |
|---|---|
| Acetaminophen 325 mg | Pain reliever/fever reducer |
| Dextromethorphan HBr 10 mg | Cough suppressant |
| Guaifenesin 100 mg | Expectorant |
| Phenylephrine HCl 5 mg | Nasal decongestant |
This product contains acetaminophen. Severe liver damage may occur if you take
If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Ask a doctor or pharmacist before use if you are taking the blood thinning drug warfarin
When using this product do not exceed recommended dosage
These could be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222). Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
| adults and children 12 years and over |
|
| children under 12 years |
|
colloidal silicon dioxide, corn starch, croscarmellose sodium, crospovidone, FD&C yellow #6, magnesium stearate, maltodextrin, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, povidone, pregelatinized starch, stearic acid, talc, titanium dioxide
Distributed by:
AAA Pharmaceutical, Inc.
681 Main Street
Lumberton, NJ 08048
RESTORE u
NDC 57344-134-03
†COMPARE TO THE ACTIVE
INGREDIENTS IN SUDAFED PE®
COLD & COUGH
NON-DROWSY
Cold & Cough PE
For Adults
Acetaminophen, Dextromethorphan HBr, Guaifenesin, Phenylephrine HCl
Pain Reliever-Fever Reducer, Cough Suppressant, Expectorant, Nasal Decongestant
Relieves: • Cough & Sore Throat • Fever & Minor Aches & Pains
Chest Congestion • Nasal & Sinus Congestion
20 CAPLETS
|
ACETAMINOPHEN, DEXTROMETHORPHAN HYDROBROMIDE, GUAIFENESIN, PHENYLEPHRINE HYDROCHLORIDE
acetaminophen, dextromethorphan hydrobromide, guaifenesin, and phenylephrine hydrochloride tablet, coated | ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC MONOGRAPH FINAL | part341 | 12/22/2012 | |
| Labeler - AAA Pharmaceutical, Inc. (181192162) |