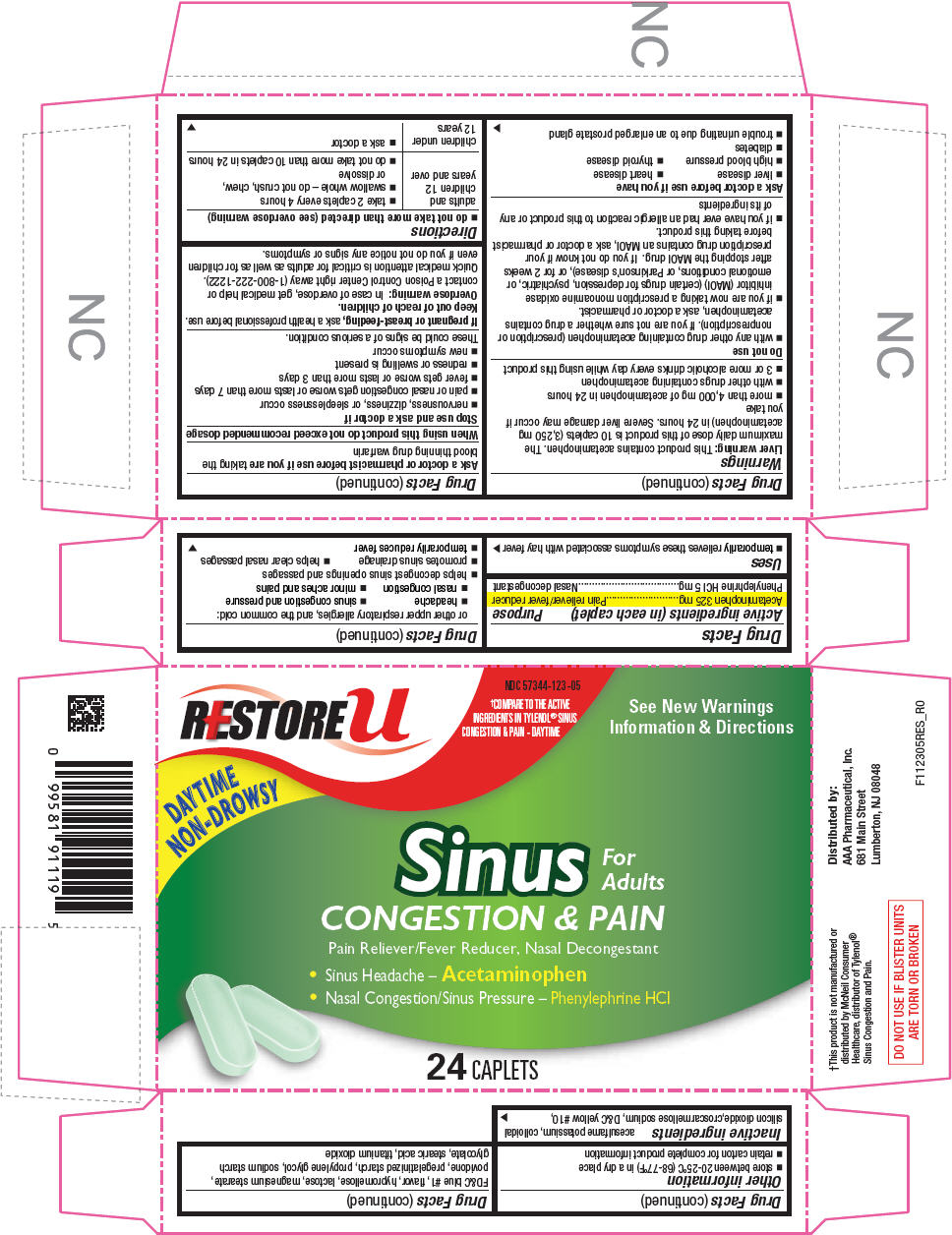
ACETAMINOPHEN, PHENYLEPHRINE HYDROCHLORIDE
-
acetaminophen and
phenylephrine hydrochloride tablet, coated
AAA Pharmaceutical, Inc.
Drug Facts
| Active ingredients (in each caplet) | Purpose |
|---|---|
| Acetaminophen 325 mg | Pain reliever/fever reducer |
| Phenylephrine HCl 5 mg | Nasal decongestant |
This product contains acetaminophen. The maximum daily dose of this product is 10 caplets (3,250 mg acetaminophen) in 24 hours. Severe liver damage may occur if you take
Do not use
Ask a doctor before use if you have
Ask a doctor or pharmacist before use if you are taking the blood thinning drug warfarin
When using this product do not exceed recommended dosage
Stop use and ask a doctor if
These could be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222). Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
| adults and children 12 years and over |
|
| children under 12 years |
|
acesulfame potassium, colloidal silicon dioxide,croscarmellose sodium, D&C yellow #10, FD&C blue #1, flavor, hypromellose, lactose, magnesium stearate, povidone, pregelatinized starch, propylene glycol, sodium starch glycolate, stearic acid, titanium dioxide
Distributed by:
AAA Pharmaceutical, Inc.
681 Main Street
Lumberton, NJ 08048
RESTORE u
NDC 57344-123-05
†COMPARE TO THE ACTIVE
INGREDIENTS IN TYLENOL® SINUS
CONGESTION & PAIN - DAYTIME
See New Warnings
Information & Directions
DAY TIME
NON-DROWSY
Sinus
For
Adults
CONGESTION & PAIN
Pain Reliever/Fever Reducer, Nasal Decongestant
24 CAPLETS
|
ACETAMINOPHEN, PHENYLEPHRINE HYDROCHLORIDE
acetaminophen and phenylephrine hydrochloride tablet, coated | ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC MONOGRAPH FINAL | part341 | 12/28/2012 | |
| Labeler - AAA Pharmaceutical, Inc. (181192162) |