ACETAMINOPHEN
-
acetaminophen tablet, coated
AAA Pharmaceutical, Inc.
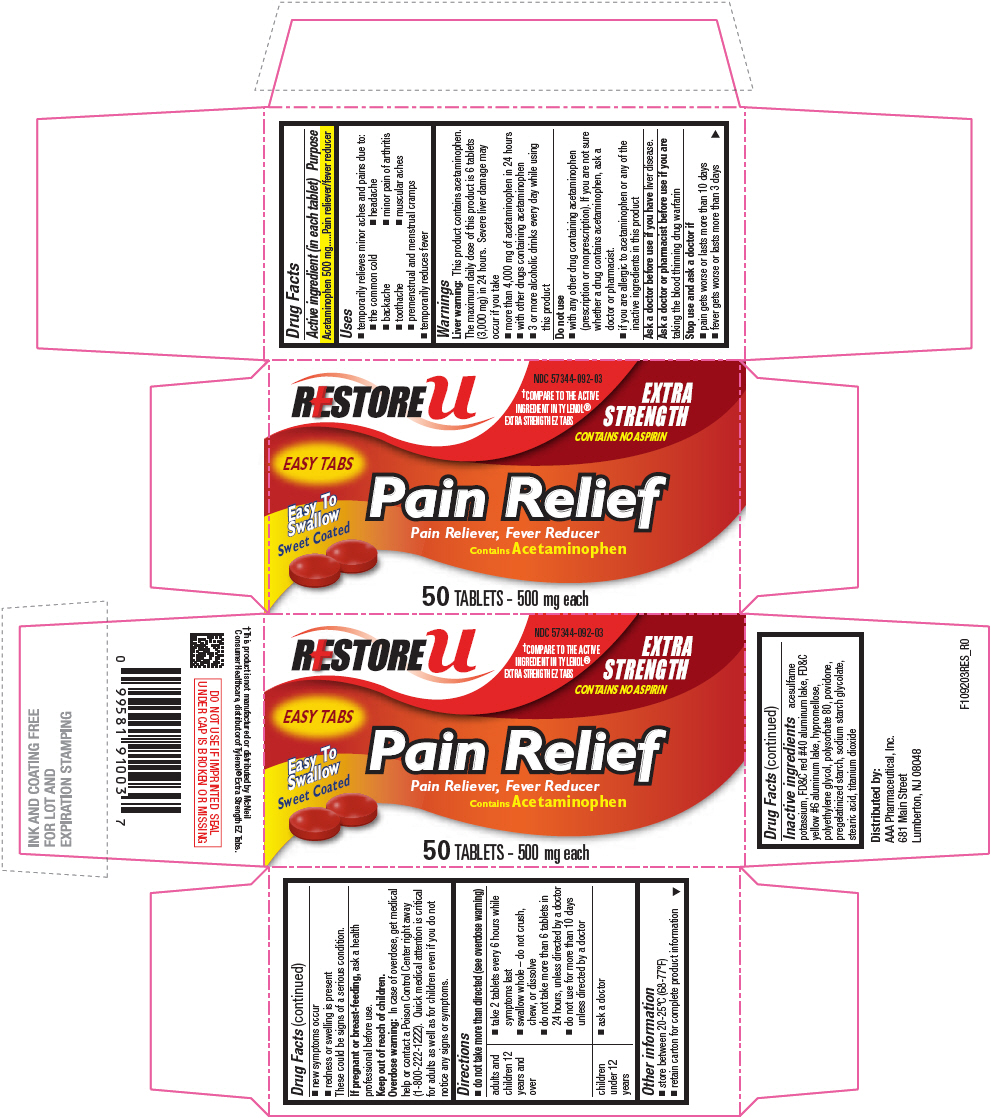
Drug Facts
Acetaminophen 500 mg
Pain reliever/fever reducer
This product contains acetaminophen. The maximum daily dose of this product is 6 tablets (3,000 mg) in 24 hours. Severe liver damage may occur if you take
Ask a doctor before use if you have liver disease.
Ask a doctor or pharmacist before use if you are taking the blood thinning drug warfarin
These could be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222). Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
| adults and children 12 years and over |
|
| children under 12 years |
|
acesulfame potassium, FD&C red #40 aluminum lake, FD&C yellow #6 aluminum lake, hypromellose, polyethylene glycol, polysorbate 80, povidone, pregelatinized starch, sodium starch glycolate, stearic acid, titanium dioxide
Distributed by:
AAA Pharmaceutical, Inc.
681 Main Street
Lumberton, NJ 08048
RESTORE u
NDC 57344-092-03
†COMPARE TO THE ACTIVE
INGREDIENT IN TYLENOL®
EXTRA STRENGTH EZ TABS
EXTRA
STRENGTH
CONTAINS NO ASPIRIN
EASY TABS
Easy To
Swallow
Sweet Coated
Pain Relief
Pain Reliever, Fever Reducer
Contains Acetaminophen
50 TABLETS - 500 mg each
|
ACETAMINOPHEN
acetaminophen tablet, coated | ||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC MONOGRAPH NOT FINAL | part343 | 12/13/2012 | |
| Labeler - AAA Pharmaceutical, Inc. (181192162) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| AAA Pharmaceutical, Inc. | 181192162 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| AAA Pharmaceutical, Inc. | 010411533 | PACK | |