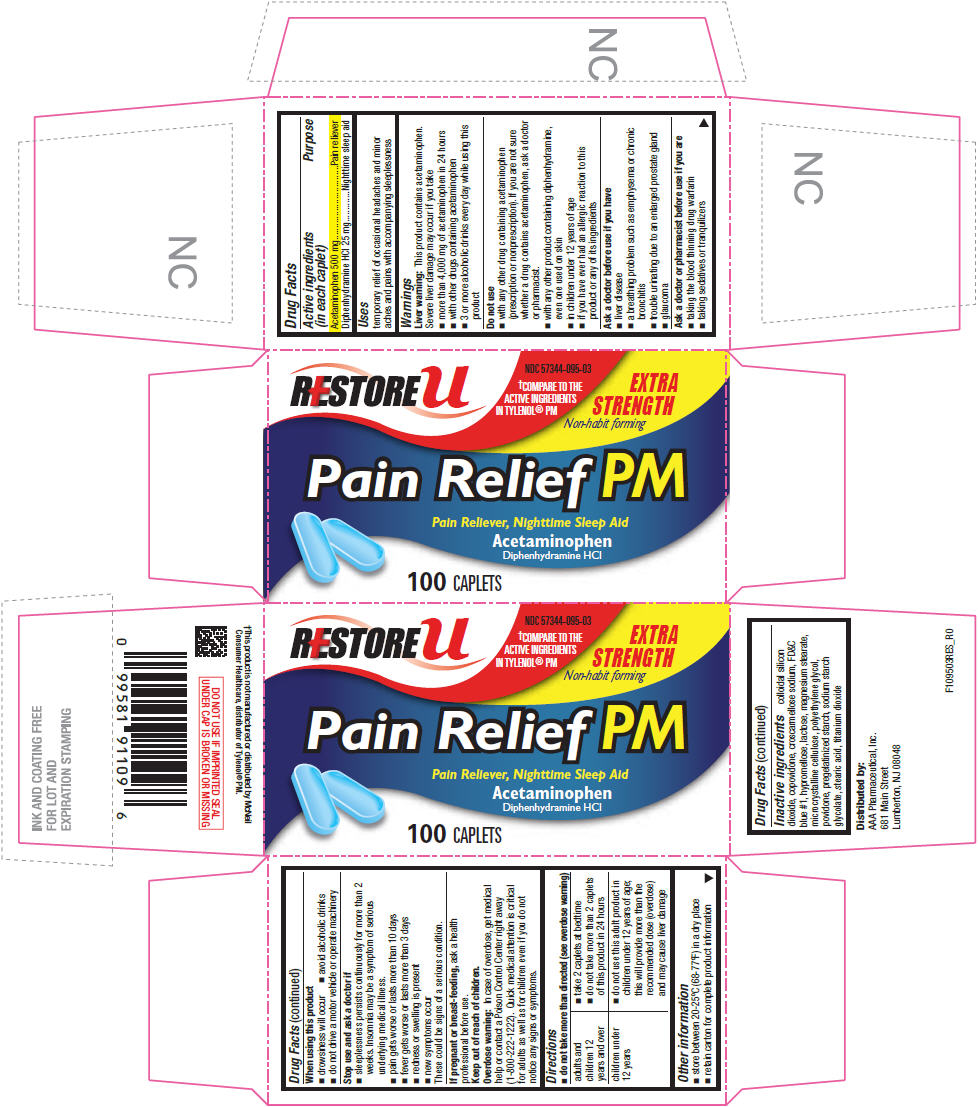
ACETAMINOPHEN PM
-
acetaminophen and
diphenhydramine hydrochloride tablet, coated
AAA Pharmaceutical, Inc.
Drug Facts
| Active ingredients (in each caplet) |
Purpose |
|---|---|
| Acetaminophen 500 mg | Pain reliever |
| Diphenhydramine HCl 25 mg | Nighttime sleep aid |
temporary relief of occasional headaches and minor aches and pains with accompanying sleeplessness
This product contains acetaminophen. Severe liver damage may occur if you take
These could be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222). Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
| adults and children 12 years and over |
|
| children under 12 years |
|
colloidal silicon dioxide, copovidone, croscarmellose sodium, FD&C blue #1, hypromellose, lactose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, pregelatinized starch, sodium starch glycolate, stearic acid, titanium dioxide
Distributed by:
AAA Pharmaceutical, Inc.
681 Main Street
Lumberton, NJ 08048
RESTORE u
NDC 57344-095-03
†COMPARE TO THE
ACTIVE INGREDIENTS
IN TYLENOL® PM
EXTRA
STRENGTH
Non-habit forming
Pain Relief PM
Pain Reliever, Nighttime Sleep Aid
Acetaminophen
Diphenhydramine HCI
100 CAPLETS
|
ACETAMINOPHEN PM
acetaminophen and diphenhydramine hydrochloride tablet, coated | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC MONOGRAPH FINAL | part338 | 12/13/2012 | |
| Labeler - AAA Pharmaceutical, Inc. (181192162) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| AAA Pharmaceutical, Inc. | 181192162 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| AAA Pharmaceutical, Inc. | 010411533 | PACK | |