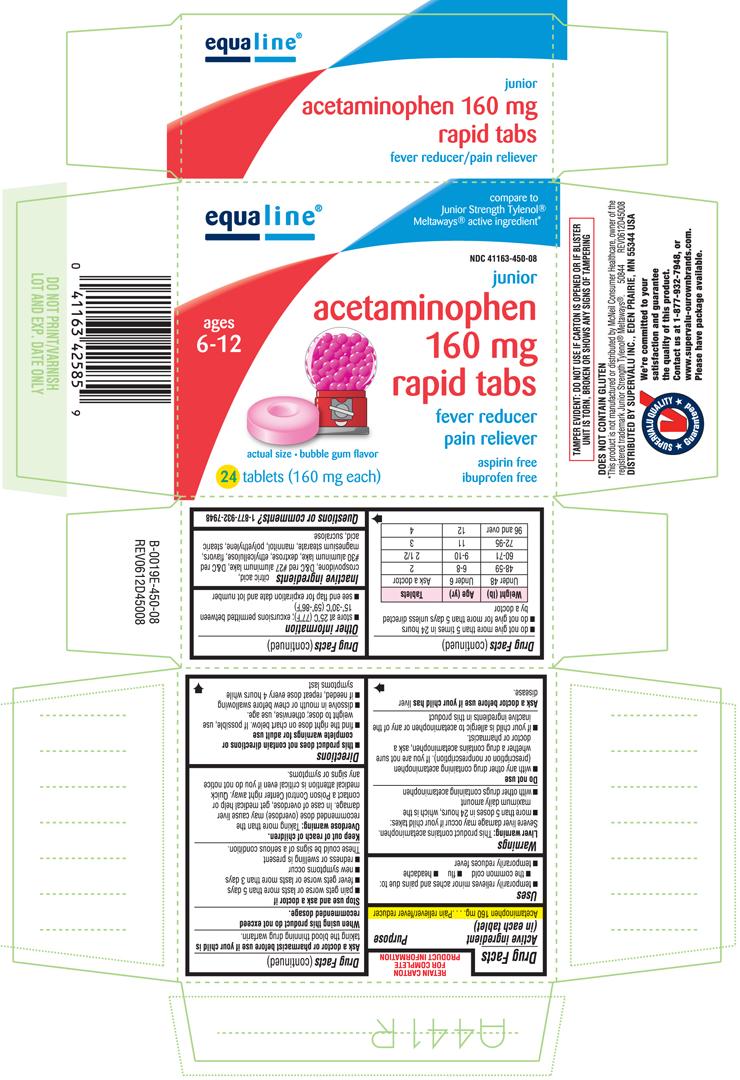
JUNIOR ACETAMINOPHEN
-
acetaminophen tablet, chewable
SUPERVALU INC.
Acetaminophen 160 mg
Pain reliever/fever reducer
Liver warning: This product contains acetaminophen. Severe liver damage may occur if your child takes:
liver disease
taking the blood thinning drug warfarin.
do not exceed the recommended dosage.
These could be signs of a serious condition.
Overdose warning: Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center right away. Quick medical attention is critical even if you do not notice any signs or symptoms.
| Weight (lb) | Age (yr) | Tablets |
| Under 48 | Under 6 | Ask a doctor |
| 48-59 | 6-8 | 2 |
| 60-71 | 9-10 | 2 1/2 |
| 72-95 | 11 | 3 |
| 96 and over | 12 | 4 |
citric acid, crospovidone, D&C red #27 aluminum lake, D&C red #30 aluminum lake, dextrose, ethylcellulose, flavors, magnesium stearate, mannitol, stearic acid, sucralose
1-877-932-7928
equaline
compare to Junior Strength Tylenol® Meltaways® active ingredients*
NDC 41163-450-08
ages
6-12
junior
acetaminophen
160 mg
rapid tabs
fever reducer
pain reliever
aspirin free
ibuprofen free
actual size • bubble gum flavor
24 tablets (160 mg each)
*This product is not manufactured or distributed by Novartis Consumer Health, Inc., owner of the registered trademark Junior Strength Tylenol® Meltaways®.
50844 REV0612D45008
DISTRIBUTED BY SUPERVALU INC.
EDEN PRARITE, MN 55344 USA
TAMPER EVIDENT: DO NOT USE IF CARTON IS OPENED OR BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
Equaline 44-450
|
JUNIOR ACETAMINOPHEN
acetaminophen tablet, chewable | ||||||||||||||||||||||||
| ||||||||||||||||||||||||
| ||||||||||||||||||||||||
| ||||||||||||||||||||||||
| ||||||||||||||||||||||||
| ||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC MONOGRAPH NOT FINAL | part343 | 02/25/2005 | |
| Labeler - SUPERVALU INC. (006961411) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 038154464 | PACK | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 832867894 | MANUFACTURE | |