FEVERALL ADULTS
-
acetaminophen suppository
Actavis Mid Atlantic LLC
FeverAll ® (Acetaminophen Suppositories) ADULTSOTC - ACTIVE INGREDIENT
Acetaminophen, USP 650 mg
OTC - PURPOSES
Pain reliever/fever reducer
USES
Temporarily
- reduces fever
- relieves minor aches, pains, and headache
WARNINGS
Liver warning: This product contains acetaminophen. Severe liver damage may occur if
- an adult or child 12 years and older takes more than 6 doses in 24 hours, which is the maximum daily amount
- taken with other drugs containing acetaminophen
- an adult takes 3 or more alcoholic drinks everyday while using this product
For rectal use only
OTC - DO NOT USE
- in children under 12 years.
- if you are allergic to acetaminophen.
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist
OTC - ASK DOCTOR
- you have liver disease.
- you are taking the blood thinning drug warfarin.
OTC - STOP USE AND ASK A DOCTOR IF
- fever lasts more than 3 days (72 hours), or recurs.
- pain gets worse or lasts more than 10 days.
- new symptoms occur.
- redness or swelling is present in the painful area.
These may be signs of a serious condition.
OTC - PREGNANCY OR BREAST FEEDING
If pregnant or breast-feeding, ask a health professional before use.
OTC - KEEP OUT OF REACH OF CHILDREN
If swallowed or in case of overdose, get medical help or contact a Poison Control Center right away. Quick medical attention is critical in case of overdose for adults and for children even if you do not notice any signs or symptoms.
DIRECTIONS
- do not use more than directed
- remove wrapper
- carefully insert suppository well up into the rectum
- adults and children 12 years and older:
- 1 suppository every 4 to 6 hours while symptoms last
- do not exceed 6 suppositories in any 24-hour period
- children under 12 years: ask a doctor
Other information
- store at 2º-27ºC (35º-80ºF)
- do not use if imprinted suppository wrapper is opened or damaged
INACTIVE INGREDIENT
Glycerol monostearate, hydrogenated vegetable oil, polyoxyethylene stearate, polysorbate 80
OTC - QUESTIONS
1-800-432-8534 between 9 am and 4 pm EST, Monday – Friday.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Adults’
Ages 12 Years And Older
NOT FOR RETAIL SALE
NDC 0472-1203-50
See New Warnings Information
FeverAll®
Acetaminophen Suppositories
Pain reliever/fever reducer
50 Rectal Suppositories
650 mg each
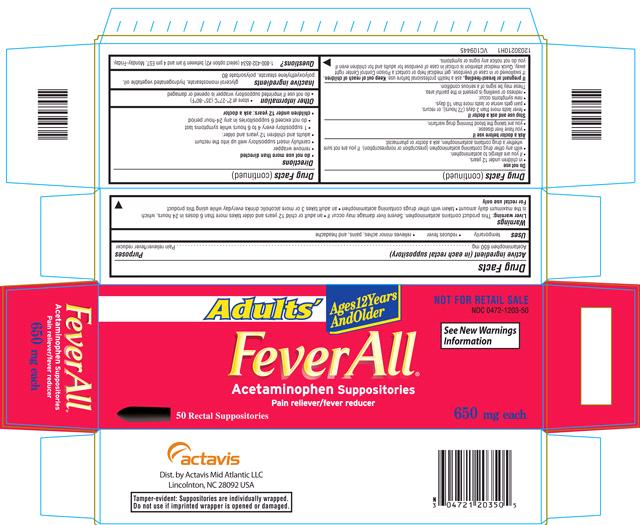
50 RECTAL SUPPOSITORIES CARTON LABEL
FEVERALL ADULTS
acetaminophen
suppository
|
|
|
|
|
|
Revised: 02/2012Actavis Mid Atlantic LLC
