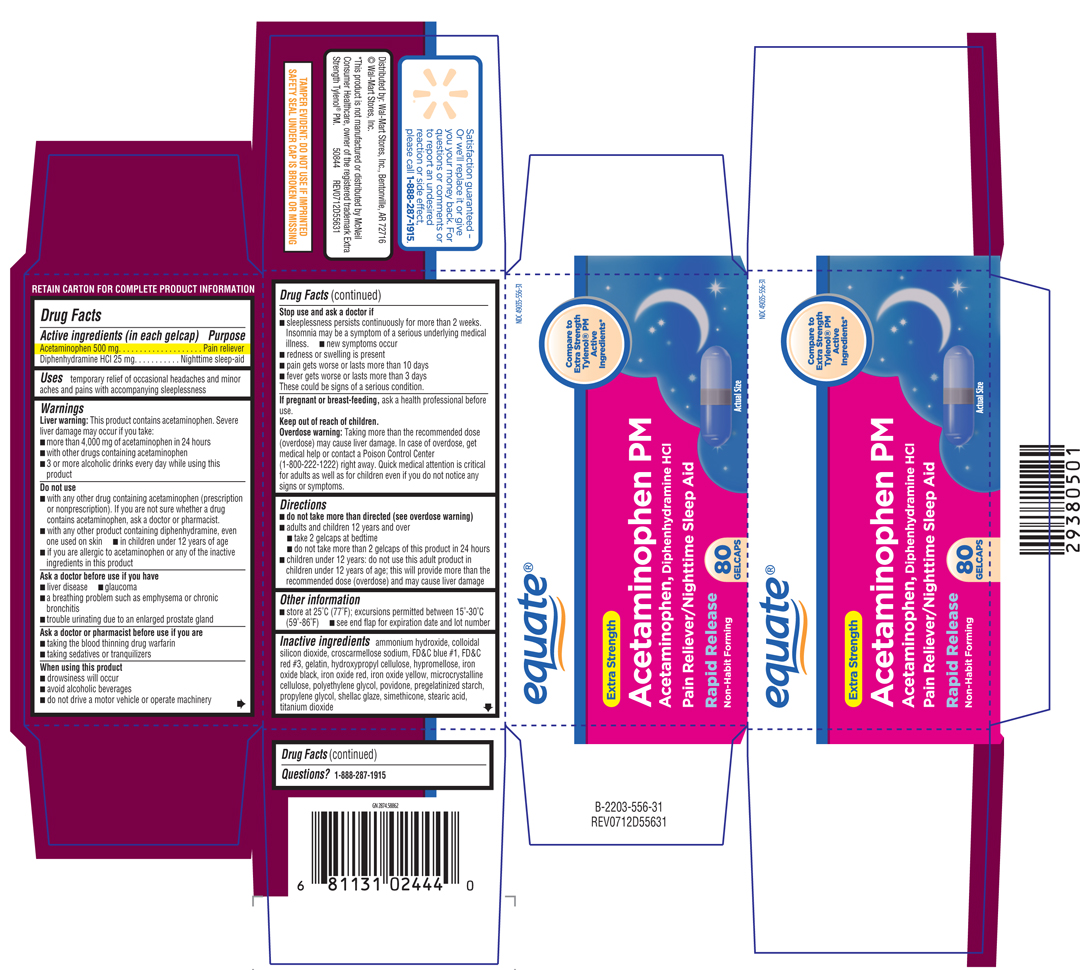
EXTRA STRENGTH ACETAMINOPHEN PM
-
acetaminophen and
diphenhydramine hydrochloride capsule
Wal-Mart Stores Inc
Acetaminophen 500 mg
Diphenhydramine HCl 25 mg
Pain reliever
Nighttime sleep-aid
temporary relief of occasional headaches and minor aches and pains with accompanying sleeplessness
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
These could be signs of a serious condition.
ask a health professional before use.
Overdose warning: Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center right away. Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
colloidal silicon dioxide, croscarmellose sodium, FD&C blue #1, FD&C red #3, gelatin, hydroxypropyl cellulose, hypromellose, iron oxide black, iron oxide red, iron oxide yellow, microcrystalline cellulose, polyethylene glycol, povidone, pregelatinied starch, propylene gllycol, shellac glaze, stearic acid, titanium dioxide
1-800-426-9391
NDC 49035-556-31
equate
Compare to The Active Ingredient of Extra Strength Tylenol® PM*
EXTRA STRENGTH
Acetaminophen PM
Acetaminophen, Diphenhydramine HCl
Pain Reliever/Nighttime Sleep-Aid
Non-habit forming
80 GELCAPS
*This product is not manufactured or distributed by McNeil Consumer Healthcare, owner of the registered trademark Extra Strength Tylenol® PM .
50844 REV0712D55631
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SEAL UNDER CAP IS BROKEN OR MISSING
Equate 44-556
|
EXTRA STRENGTH ACETAMINOPHEN PM
acetaminophen and diphenhydramine hcl capsule | ||||||||||||||||||||||||
| ||||||||||||||||||||||||
| ||||||||||||||||||||||||
| ||||||||||||||||||||||||
| ||||||||||||||||||||||||
| ||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC MONOGRAPH NOT FINAL | part341 | 12/17/2007 | |
| Labeler - Wal-Mart Stores Inc (051957769) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 038154464 | PACK | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 832867894 | MANUFACTURE | |