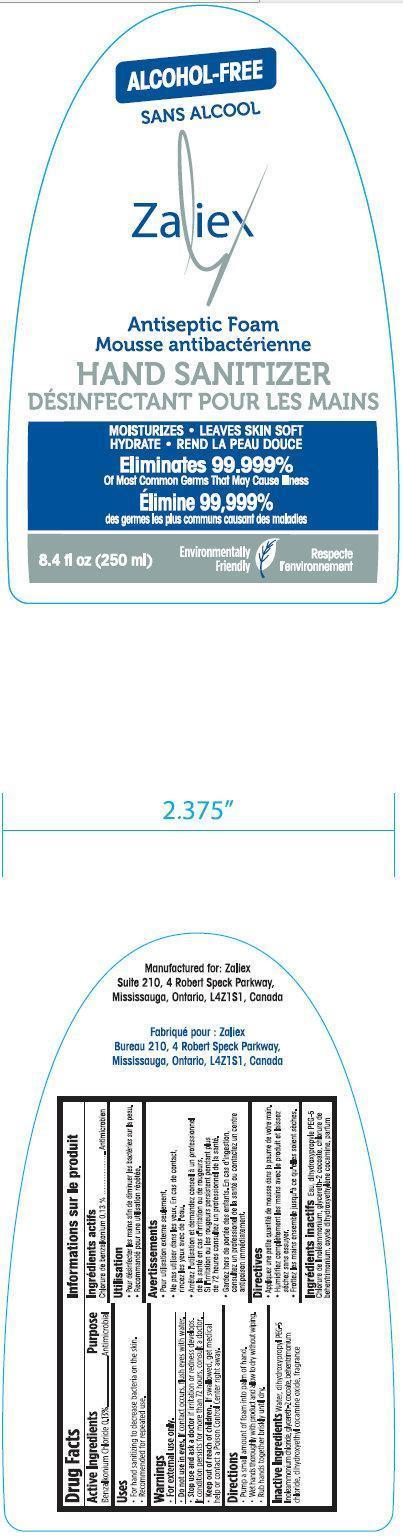
ZALIEX ALCOHOL-FREE ANTISEPTIC HAND SANITIZER
-
benzalkonium chloride liquid
SAS Healthcare Inc
Benzalkonium Chloride 0.13%
Antimicrobial
For external use only.
Do not use in eyes. If contact occurs, flush eyes with water.
Stop use and ask a doctor if irritation or redness develops.
If condition persists for more than 72 hours, consult a doctor.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Water, dihydroxypropyl PEG-5 linoleammonium chloride, glycereth-2 cocoate, behentrimonium chloride, dihydroxyethyl cocamine oxide, fragrance
Zaliex
Suite 210, 4 Robert Speck Parkway,
Mississauga, Ontario, L4Z1S1, Canada

|
ZALIEX ALCOHOL-FREE ANTISEPTIC HAND SANITIZER
benzalkonium chloride liquid | ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part333E | 09/13/2012 | |
| Labeler - SAS Healthcare Inc (248055696) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Artemis Bio-Solutions Inc. | 963442541 | manufacture | |