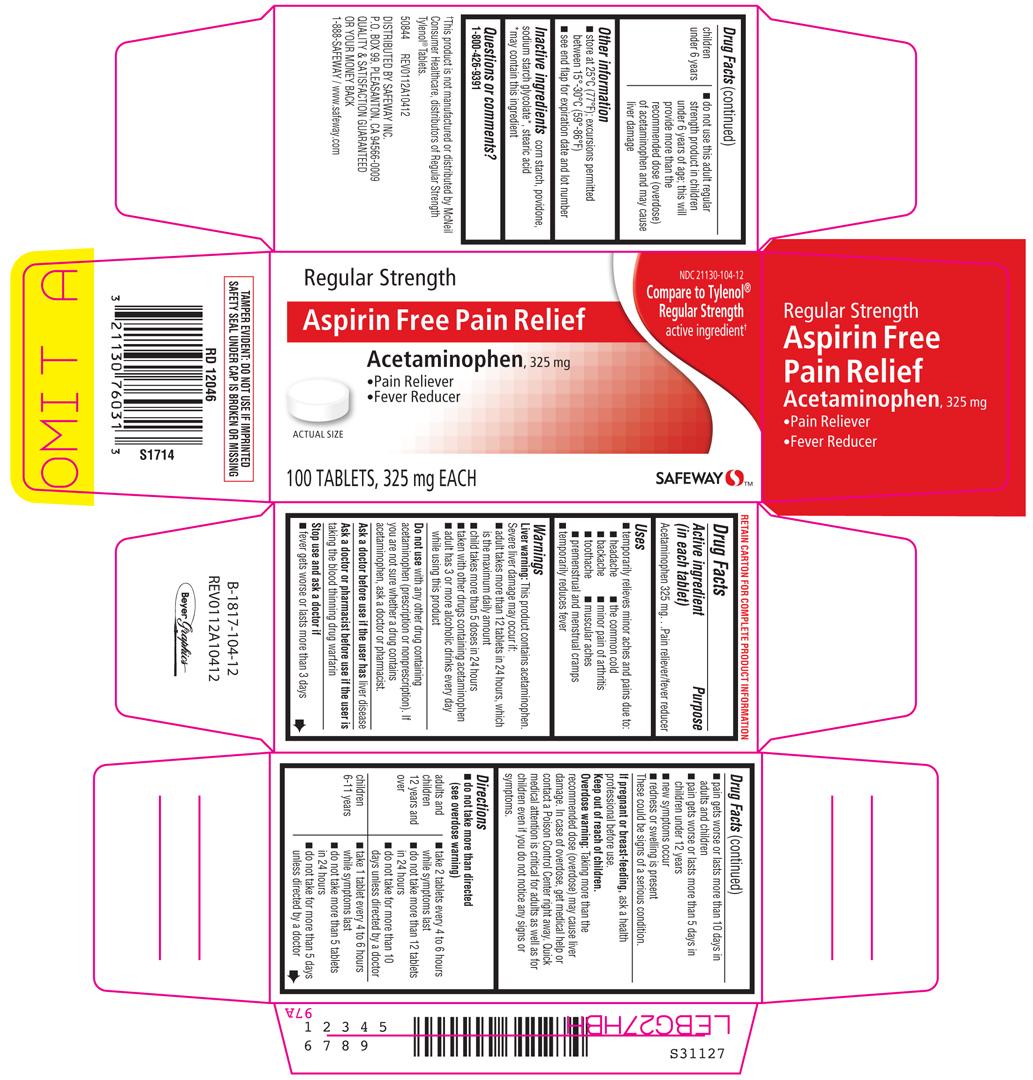
ACETAMINOPHEN
-
acetaminophen tablet
Safeway
Acetaminophen 325 mg
Pain reliever/fever reducer
Liver Warning: This product contains acetaminophen. Severe liver damage may occur if:
with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
liver disease
taking the blood thinning drug warfarin
These could be signs of a serious condition.
ask a health professional before use.
Overdose warning: Taking more than the recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center right away. Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
| adults and children 12 years and over |
|
| children 6-11 years |
|
| children under 6 years |
|
corn starch, povidone, sodium starch glycolate*, stearic acid
*may contain this ingredient
1-800-426-9391
Regular Strength
NDC 21130-104-12
Compare to Tylenol® Regular Strength active ingredient†
Aspirin Free Pain Relief
Acetaminophen, 325 mg
• Pain Reliever
• Fever Reducer
ACTUAL SIZE
100 TABLETS, 325 mg EACH
SAFEWAY™
†This product is not manufactured or distributed by McNeil Consumer Healthcare, owner of the registered trademark Regular Strength Tylenol® Tablets.
50844 REV0112A10412
DISTRIBUTED BY SAFEWAY, INC.
P.O. BOX 99, PLEASANTON, CA 94566-0009
QUALITY & SATISFACTION GUARANTEED
OR YOUR MONEY BACK
1-888-SAFEWAY / www.safeway.com
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
Safeway 44-104
|
ACETAMINOPHEN
acetaminophen tablet | ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC MONOGRAPH FINAL | part343 | 07/13/1990 | |
| Labeler - Safeway (009137209) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 832867894 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 038154464 | PACK | |