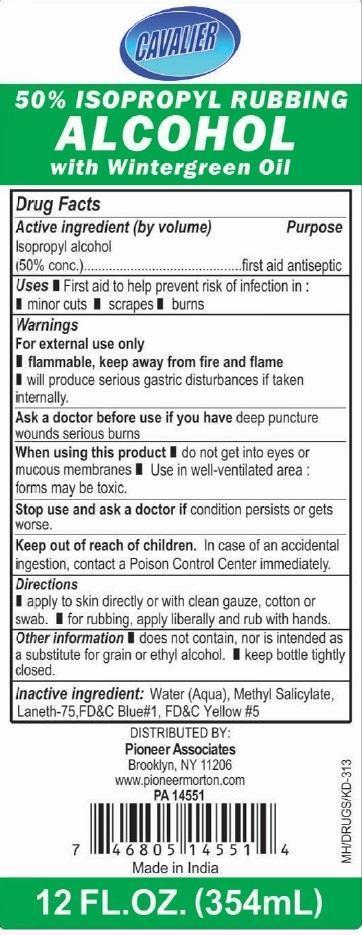
ISOPROPYL RUBBING ALCOHOL WITH WINTERGREEN OIL
-
isopropyl alcohol liquid
Pioneer Associates
Active ingredient (by volume)
Isopropyl alcohol (50% conc.)
Purpose
first aid antiseptic
Uses
Warnings
For external use only
Ask a doctor before use if you have deep puncture wounds or serious burns
When using this product
Stop use and ask a doctor if condition persists or gets worse
Keep out of reach of children.
In case of an accidental ingestion, contact a Poison Control Center immediately
Directions
Other information
Inactive ingredient
Water (Aqua), methyl salicylate, laneth-75, FD&C Blue #1, FD&C Yellow #5
PRINCIPAL DISPLAY PANEL
CAVALIER ISOPROPYL RUBBING ALCOHOL 50% WITH WINTERGREEN OIL
FIRST AID ANTISEPTIC
12 FL.OZ (354 mL)
|
ISOPROPYL RUBBING ALCOHOL WITH WINTERGREEN OIL
isopropyl alcohol liquid | ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part333A | 08/24/2012 | |
| Labeler - Pioneer Associates (012604336) |
| Registrant - Anicare Pharmaceuticals Pvt. Ltd (916837425) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Anicare Pharmaceuticals Pvt. Ltd | 916837425 | manufacture | |