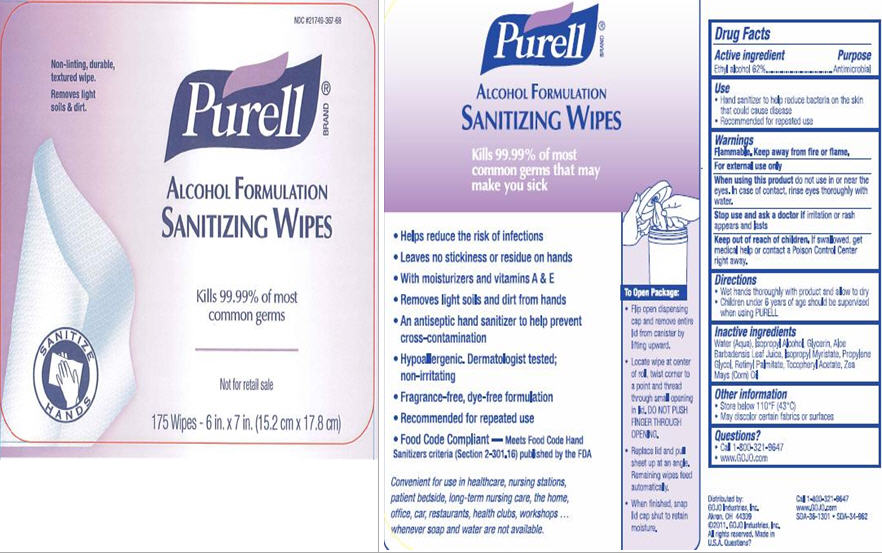
PURELL ALCOHOL FORMULATION
-
alcohol swab
GOJO Industries, Inc.
Ethyl Alcohol 62%
Antimicrobial
Flammable. Keep away from fire or flame.
For external use only
do not use in or near the eyes. In case of contact, rinse eyes thoroughly with water.
if irritation or rash appears and lasts
If swallowed, get medical help or contact a Poison Control Center right away.
Water (Aqua), Isopropyl Alcohol, Glycerin, Aloe Barbadensis Leaf Juice, Isopropyl Myristate, Propylene Glycol, Retinyl Palmitate, Tocopheryl Acetate, Zea Mays (Corn) Oil
NDC #21749-367-68
Non-linting, durable, textured wipe.
Removes light soils & dirt.
Purell
ALCOHOL FORMLATION
SANITIZING WIPES
Kills 99.99% of most common germs
Not for retail sale
175 Wipes - 6 in. x 7 in. (15.2 cm x 17.8 cm)
Canister Label
|
PURELL ALCOHOL FORMULATION
alcohol swab | ||||||||||||||||||||||
| ||||||||||||||||||||||
| ||||||||||||||||||||||
| ||||||||||||||||||||||
| ||||||||||||||||||||||
| ||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part333E | 02/24/2012 | |
| Labeler - GOJO Industries, Inc. (004162038) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| GOJO Industries, Inc. | 036424534 | MANUFACTURE | |