XERESE
-
acyclovir and
hydrocortisone cream
Valeant Pharmaceuticals North America LLC
| |||||||||||||||||||||
XERESE is indicated for the early treatment of recurrent herpes labialis (cold sores) to reduce the likelihood of ulcerative cold sores and to shorten the lesion healing time in adults and adolescents (12 years of age and older).
Topically apply XERESE 5 times per day for 5 days. Therapy should be initiated as early as possible after the first signs and symptoms (i.e., during the prodrome or when lesions appear).
For each dose, topically apply a quantity of XERESE sufficient to cover the affected area, including the outer margin. Avoid unnecessary rubbing of the affected area to avoid aggravating or transferring the infection. For adolescents 12 years of age and older, the dosage is the same as in adults.
Each gram of XERESE contains 5% (w/w) acyclovir and 1% (w/w) hydrocortisone in an aqueous cream base.
None.
XERESE is intended for cutaneous use only for herpes labialis of the lips and around the mouth. XERESE should not be used in the eye, inside the mouth or nose, or on the genitals.
There are other orofacial lesions, including bacterial and fungal infections, which may be difficult to distinguish from a cold sore. Patients should be encouraged to seek medical advice when a cold sore fails to heal within 2 weeks.
XERESE has a potential for irritation and contact sensitization [see Adverse Reactions (6)].
The safety data derived from XERESE clinical studies reflect exposure to XERESE in 1002 subjects with recurrent herpes labialis treated 5 times daily for 5 days. The majority of the adverse reactions were local skin reactions and occurred in the area of the application site.
Because clinical studies are conducted under widely varying conditions, the adverse reaction rates observed cannot be directly compared to rates in other clinical studies and may not reflect the rates observed in clinical practice.
The majority of the adverse reactions were local and occurred in the area of the application site.
Skin and Subcutaneous Tissue Disorders
The following most common adverse reactions (<1%) were local skin reactions, and occurred in the area of the application site:
Contact dermatitis following application has been observed when applied under occlusion in dermal safety studies. Where contact sensitivity tests have been conducted, the reactive substances were hydrocortisone or a component of the cream base.
A study enrolling 225 healthy adults was conducted to evaluate the contact sensitization potential of XERESE using repeat insult patch testing methodology. Of 205 evaluable subjects, one confirmed case (0.5%) of sensitization to hydrocortisone and 2 additional cases (1.0%) of possible sensitization to the XERESE base were identified. Additionally, one subject developed a contact allergy in the photosafety study to propylene glycol, one of the inactive ingredients of the cream base.
Dermal tolerance was assessed in a 21-day cumulative irritation study in 36 healthy subjects. XERESE, its cream base and Zovirax® (acyclovir) Cream 5% all showed a high and cumulative irritation potential under occlusive and semiocclusive conditions.
Photoallergic potential and phototoxicity were assessed in two studies in 50 and 30 healthy volunteers, respectively. No photoallergic or phototoxicity potential was identified for XERESE.
No drug interaction studies have been performed with XERESE.
Category B
Teratogenic Effects
Acyclovir was not teratogenic in the mouse, rabbit or rat at exposures greatly in excess of human exposure. There are no adequate and well-controlled studies of systemic acyclovir in pregnant women. A prospective epidemiologic registry of acyclovir use during pregnancy between 1984 and 1999 followed 749 pregnancies in women exposed to systemic acyclovir during the first trimester of pregnancy resulting in 756 outcomes. The occurrence rate of birth defects approximated that found in the general population. However, the size of the registry was insufficient to evaluate the risk for less common defects or to permit reliable or definitive conclusions regarding the safety of acyclovir in pregnant women and their developing fetuses.
Corticosteroids are generally teratogenic in laboratory animals when administered systemically at relatively low dosage levels. The more potent corticosteroids have been shown to be teratogenic after dermal application in laboratory animals.
Animal reproduction studies have not been conducted with XERESE. No studies have been performed in pregnant women. Systemic exposure of acyclovir and hydrocortisone following topical administration of XERESE is minimal.
It is not known whether topically applied acyclovir or hydrocortisone is excreted in breast milk. Systemic exposure following topical administration of either drug is expected to be below detection limits. Because many drugs are excreted in human milk, caution should be exercised when XERESE is administered to a nursing woman.
Safety and effectiveness in pediatric subjects less than 12 years of age have not been established.
In clinical studies, there were insufficient subjects above 65 years of age to reach a firm conclusion regarding safety and efficacy of XERESE in this group, although the available results were similar to lower age subjects.
Even though the safety of XERESE has been studied in immunocompromised subjects, data are insufficient to support use in this population. Immunocompromised subjects should be encouraged to consult a physician concerning the treatment of any infection.
Benefit has not been adequately assessed in immunocompromised patients. A randomized, double-blind study was conducted in 107 immunocompromised subjects with stable HIV infection and recurrent herpes labialis. Subjects had on average 3.7 episodes of herpes labialis in the previous 12 months. The median age was 30 years (range 19 to 64 years), 46% were female, and all Caucasian. Median CD4+ T-cell count at screening was 344/mm3 (range 100-500/mm3). Subjects were treated with XERESE or 5% acyclovir in XERESE vehicle. The primary objective was to exclude a doubling of the healing time in either treatment arm. The mean healing time for cold sores was similar between the two treatment groups: 6.6 days for XERESE and 6.9 days for 5% acyclovir in XERESE vehicle.
Overdosage by topical application of XERESE is unlikely because of minimal systemic exposure [see Clinical Pharmacology (12.3)].
XERESE contains acyclovir, a synthetic nucleoside analogue active against herpes viruses, and hydrocortisone, an anti-inflammatory corticosteroid, combined in a cream for topical administration. Each gram of XERESE contains 5% (w/w) of acyclovir, 1% (w/w) of hydrocortisone and the following inactive ingredients: cetostearyl alcohol, mineral oil, Poloxamer 188, propylene glycol, isopropyl myristate, sodium lauryl sulfate, white petrolatum, citric acid, sodium hydroxide and water. Sodium hydroxide or hydrochloric acid may be added to adjust the pH to approximately pH 5.
Acyclovir, 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6H-purin-6-one, is a synthetic nucleoside analogue active against herpes viruses. The maximum solubility of acyclovir in water at 37°C is 2.5 mg/mL. The pKa’s of acyclovir are 2.27 and 9.25. Its empirical formula is C8H11N5O3. The structural formula is provided in Figure 1:
Figure 1: Structural Formula of Acyclovir
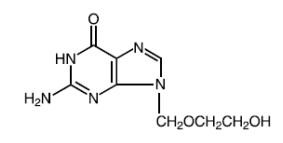
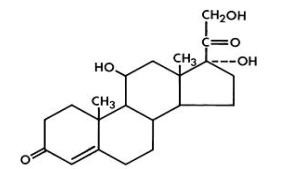
Hydrocortisone, pregn-4-ene-3, 20-dione, 11, 17, 21-trihydroxy-(11(beta))-, is an anti-inflammatory corticosteroid. Its empirical formula is C21H30O5. The structural formula is provided in Figure 2:
Figure 2: Structural Formula of Hydrocortisone
Acyclovir is an antiviral drug and hydrocortisone an anti-inflammatory drug. [see Clinical Pharmacology (12.4)].
The plasma concentrations of acyclovir and hydrocortisone were not measured following topical administration of XERESE on cold sores.
The extent of percutaneous absorption of topical corticosteroids is determined by many factors including the vehicle, the integrity of the epidermal barrier, and the use of occlusive dressings.
Topical corticosteroids can be absorbed from normal intact skin and can have systemic side effects depending on both the potency of the corticosteroid and the surface area of application. Inflammation and/or other disease processes in the skin that disrupt the skin barrier can increase percutaneous absorption.
Once absorbed through the skin, topical corticosteroids are handled through pharmacokinetic pathways similar to systemically administered corticosteroids. Corticosteroids are bound to plasma proteins in varying degrees. They are metabolized primarily in the liver and are then excreted by the kidneys. Some of the topical corticosteroids and their metabolites are also excreted into the bile.
Mechanism of Action
Acyclovir is a synthetic purine nucleoside analogue with inhibitory activity against herpes simplex viruses type 1 (HSV-1) and type 2 (HSV-2) in cell culture and in vivo.
The inhibitory activity of acyclovir is highly selective due to its affinity for the enzyme thymidine kinase (TK) encoded by HSV. This viral enzyme converts acyclovir into acyclovir monophosphate, a nucleotide analogue. The monophosphate is further converted into diphosphate by cellular guanylate kinase and into triphosphate by a number of cellular enzymes. In cell culture, acyclovir triphosphate stops replication of herpes viral DNA. This inhibition is accomplished in 3 ways: 1) competitive inhibition of viral DNA polymerase, 2) incorporation into and termination of the growing viral DNA chain, and 3) inactivation of the viral DNA polymerase.
Hydrocortisone is the main glucocorticoid secreted by the adrenal cortex. It is used topically for its anti-inflammatory effects which suppress the clinical manifestations of the disease in a wide range of disorders where inflammation is a prominent feature.
Antiviral Activity
The quantitative relationship between the cell culture susceptibility of herpes viruses to antivirals and the clinical response to therapy has not been established in humans, and virus sensitivity testing has not been standardized. Sensitivity testing results, expressed as the concentration of drug required to inhibit by 50% the growth of virus in cell culture (EC50), vary greatly depending upon a number of factors. Using plaque reduction assays on Vero cells, the median EC50 value of acyclovir against clinical herpes virus isolates (subjects receiving placebo) was 1.3 µM (range: <0.56 to 3.3 µM).
Resistance
Resistance of HSV to acyclovir can result from qualitative and quantitative changes in the viral TK and/or DNA polymerase. Clinical isolates of HSV with reduced susceptibility to acyclovir have been recovered from immunocompromised subjects, especially with advanced HIV infection. While most of the acyclovir resistant mutants isolated from immunocompromised subjects thus far have been found to be TK-deficient mutants, other mutants involving the viral TK gene (TK partial and TK altered) and DNA polymerase have been isolated. TK-negative mutants may cause severe disease in infants and immunocompromised adults.
The possibility of viral resistance to acyclovir should be considered in patients who show poor clinical response during therapy.
Systemic exposure following topical administration of acyclovir is minimal. Results from previous studies of carcinogenesis, mutagenesis and fertility for acyclovir and hydrocortisone are not included in the full prescribing information for XERESE due to the minimal exposures that result from dermal application. Information on these studies following systemic exposure is available in the full prescribing information for acyclovir and hydrocortisone products approved for oral or parenteral administration. Dermal carcinogenicity studies have not been conducted.
In a double-blind, clinical study, 1443 subjects with recurrent labial herpes were randomized to receive XERESE, 5% acyclovir in XERESE vehicle or vehicle alone. Subjects had, on average, 5.6 episodes of herpes labialis in the previous 12 months. The median age was 44 years (range 18 to 80 years), 72% were female, and 91% were Caucasian. Subjects were instructed to initiate treatment within 1 hour of noticing signs or symptoms and continue treatment for 5 days, with application of study medication 5 times per day. Ulcerative cold sores occurred in 58% of the subjects treated with XERESE compared to 74% in subjects treated with vehicle and 65% in subjects treated with 5% acyclovir in XERESE vehicle. The mean time to skin normalization was approximately 1.6 days shorter in the subjects treated with XERESE compared to vehicle. Clinical signs in terms of size of the cold sore and symptoms such as tenderness were reduced with XERESE as compared to vehicle.
An open label safety study in adolescents with recurrent herpes labialis was conducted in 134 subjects. Subjects had, on average, 4.0 episodes of herpes labialis in the previous 12 months. The median age was 14 years (range 12 to 17 years); 50% were female and all were Caucasian. Therapy was applied using the same dosing regimen as in adults and subjects were monitored for adverse events and selected efficacy parameters. The safety and efficacy profile appeared similar to that observed in adults.
XERESE is supplied in a plastic-laminated aluminum tube containing 5 gm of XERESE. Each gram of XERESE contains 5% (w/w) acyclovir and 1% (w/w) hydrocortisone in an aqueous cream base.
NDC 0187-5104-01: 5 gm tubes
Store at controlled room temperature 20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F). Do not freeze.
See FDA-Approved Patient Labeling
Patients should be informed that XERESE is not a cure for cold sores. Patients should be instructed that XERESE is intended for cutaneous use only for herpes labialis of the lips and around the mouth. Patients should be advised that XERESE should not be used in the eye, inside the mouth or nose, or on the genitals.
Patients should be advised to apply XERESE topically 5 times per day for 5 days. Patients should be instructed to topically apply a quantity of XERESE sufficient to cover the affected area, including the outer margin. Patients should be advised to avoid unnecessary rubbing of the affected area to avoid aggravating or transferring the infection.
FDA-Approved Patient Labeling
PATIENT INFORMATION
XERESE® (acyclovir and hydrocortisone) Cream 5%/1%
XERESE is for cold sores on lips and around the mouth only. XERESE should not be used in eyes, mouth, nose or on genitals.
Read this Patient Information that comes with XERESE before you start using it and each time you get a refill. There may be new information. This patient leaflet does not take the place of talking with your doctor about your medical condition or treatment.
What is XERESE?
XERESE is a prescription medicine used in people ages 12 and older to lessen the healing time of cold sores (herpes labialis) and lessen the chance of a cold sore becoming worse (ulcerating).
XERESE is not a cure for cold sores.
It is not known if XERESE is safe or works in children younger than 12 years old.
What should I tell my doctor before using XERESE?
Before you use XERESE, tell your doctor if you:
How should I use XERESE?
What are the possible side effects of XERESE?
The most common side effects of XERESE are:
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of XERESE. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to Valeant Pharmaceuticals North America LLC at 1-800-321-4576 or to the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
How should I store XERESE?
Keep XERESE and all medicines out of the reach of children.
General Information about XERESE
Medicines are sometimes prescribed for conditions that are not mentioned in patient leaflets. Do not use XERESE for a condition for which it was not prescribed. Do not give XERESE to other people, even if they have the same symptoms you have. It may harm them.
This patient leaflet summarizes the most important information about XERESE. If you would like to know more information about XERESE, talk with your doctor. You can ask your doctor or pharmacist for additional information about XERESE that was written for healthcare professionals.
What are the ingredients of XERESE?
Active Ingredients: acyclovir and hydrocortisone
Inactive ingredients: cetostearyl alcohol, mineral oil, Poloxamer 188, propylene glycol, isopropyl myristate, sodium lauryl sulfate, white petrolatum, citric acid, sodium hydroxide and purified water. May also contain hydrochloric acid.
Revised: 01/2012
Manufactured for:
Valeant Pharmaceuticals North America LLC
Bridgewater, NJ 08807
Manufactured by:
Contract Pharmaceuticals Limited (CPL)
7600 Danbro Crescent
Mississauga, Ontario
Canada L5N 6L6
Produced under license
MEDA Pharma SARL, Luxembourg, by Valeant International (Barbados) SRL, Barbados, West Indies
Made in Canada
XERESE is a registered trademark of Meda Pharma SARL under license by Valeant.
U.S. Patents RE39,264 and 7,223,387
2005525
Principal Display Panel - 5 g Carton - Top Panel
XERESE®
(Acyclovir and Hydrocortisone)
Cream 5%/1%
Contains 5 g of XERESE® Cream; each gram contains 5% of acyclovir and 1% of hydrocortisone.
NDC-0187-5104-01
5g
Rx Only
For Topical Use Only
Store at controlled room temperature [20°-25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F). Do not freeze.]
Usual Dosage: Apply cream topically to the affected area 5 times daily for 5 days. See package insert for Dosing and Administration.
2005227
Rev. 01/2012

Principal Display Panel - 5 g Carton - Bottom Panel
XERESE®
(Acyclovir and Hydrocortisone)
Cream 5%/1%
Manufactured by Contract Pharmaceuticals Limited (CPL), Mississauga, ON, Canada L5N 6L6.
Produced under license from MEDA Pharm SARL, Luxembourg, by Valeant International (Barbados) SRL, Barbados, West Indies, Made in Canada
U.S. Patents RE39,264; and 7,223,387
Xerese is a registered trademark of Meda Pharma SARL used under license by Valeant.
NDC-0187-5104-01
5g
Rx Only
For Topical Use Only

|
XERESE
acyclovir and hydrocortisone cream | ||||||||||||||||||||||||
| ||||||||||||||||||||||||
| ||||||||||||||||||||||||
| ||||||||||||||||||||||||
| ||||||||||||||||||||||||
| ||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| NDA | NDA022436 | 07/15/2010 | |
| Labeler - Valeant Pharmaceuticals North America LLC (042230623) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Contract Pharmaceuticals Limited Canada | 248761249 | MANUFACTURE | |