ALCOHOL
-
isopropyl alcohol swab
School Health Corporation
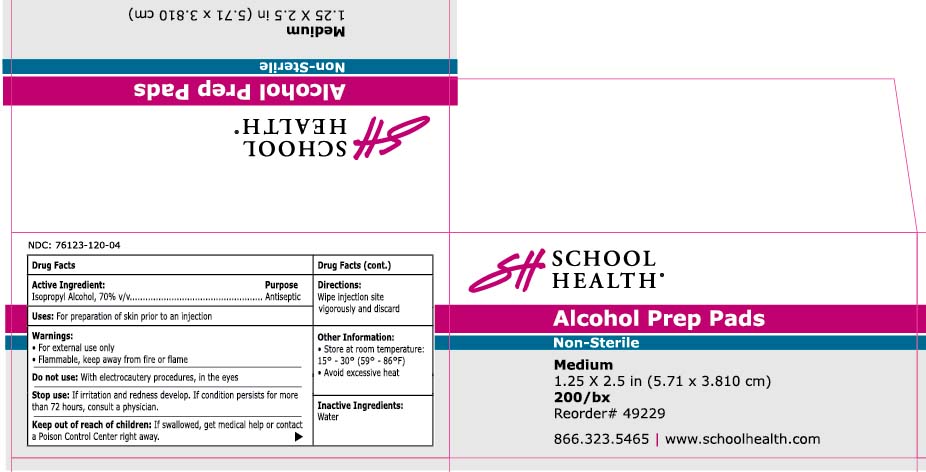
Active Ingredient Purpose
Isopropyl Alcohol 70% v/v Antiseptic
alcohol prep pad
For preparation of the skin prior to injection.
Stop use and ask a doctor if:
In case of accidental ingestion, seek professional assistance or consult a poison control center immediately.
Wipe injection site vigorously and discard
Inactive Ingredient
Alcohol Prep Pad
|
ALCOHOL
isopropyl alcohol swab | ||||||||||||||||||||
| ||||||||||||||||||||
| ||||||||||||||||||||
| ||||||||||||||||||||
| ||||||||||||||||||||
| ||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part344 | 04/05/2023 | |
| Labeler - School Health Corporation (024906331) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Shanghai Yinjing Medical SuppliesCo., Ltd. | 530501535 | manufacture | |