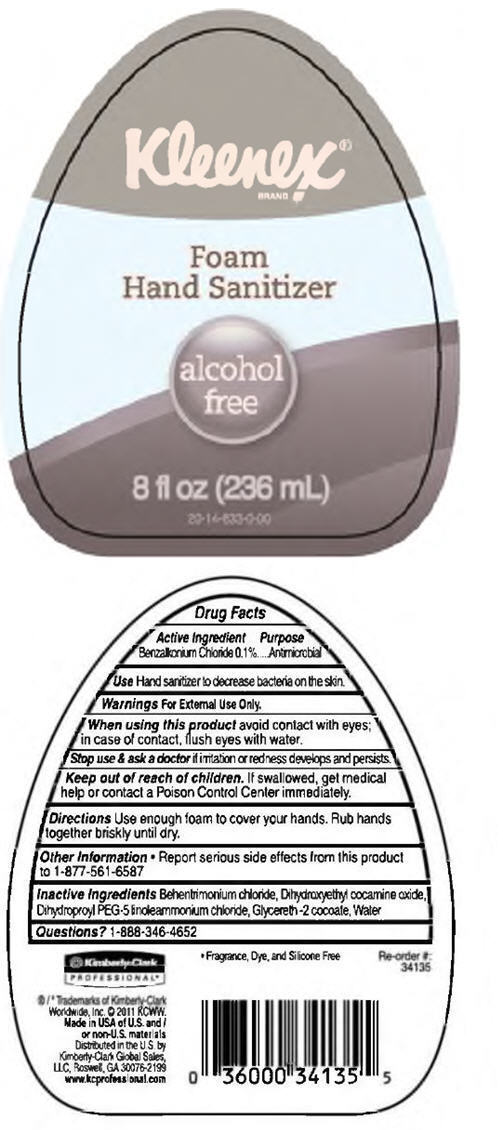
KLEENEX FOAM HAND SANITIZER, ALCOHOL FREE
-
benzalkonium chloride solution
Kimberly-Clark Corporation
Drug Facts
Benzalkonium Chloride 0.1%
Antimicrobial
Hand sanitizer to decrease bacteria on the skin.
For External Use Only.
When using this product avoid contact with eyes; in case of contact, flush eyes with water.
Stop use & ask a doctor if irritation or redness develops and persists.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Use enough foam to cover your hands. Rub hands together briskly until dry.
Behentrimonium chloride, Dihydroxyethyl cocamine oxide, Dihydroproyl PEG-5 linoleammonium chloride, Glycereth -2 cocoate, Water
1-888-346-4652
Distributed in the U.S. by
Kimberly-Clark Global Sales,
LLC, Roswell, GA 30076-2199
Kleenex®
BRAND
Foam
Hand Sanitizer
alcohol
free
8 fl oz (236 mL)
20-14-633-0-00
|
KLEENEX FOAM HAND SANITIZER, ALCOHOL FREE
benzalkonium chloride solution | ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC MONOGRAPH NOT FINAL | part333E | 03/01/2023 | |
| Labeler - Kimberly-Clark Corporation (006072136) |