FEVERALL CHILDREN
-
acetaminophen suppository
Actavis Mid Atlantic LLC
Acetaminophen 120 mg
Pain reliever/fever reducer
Temporarily
Liver warning: This product contains acetaminophen. Severe liver damage may occur if your child takes
For rectal use only
These may be signs of a serious condition.
If swallowed or in case of overdose, get medical help or contact a Poison Control Center right away. Quick medical attention is critical in case of overdose for adults and for children even if you do not notice any signs or symptoms.
| Dosing Chart | |
| Age | Dose |
| under 3 years | Do not use unless directed by a doctor |
| 3 to 6 years | Use 1 suppository every 4 to 6 hours (maximum of 5 doses in 24 hours) |
Other information
Glycerol monostearate, hydrogenated vegetable oil, polyoxyethylene stearate, polysorbate 80
1-800-432-8534 (select option #2) between 9 am and 4 pm EST, Monday – Friday.
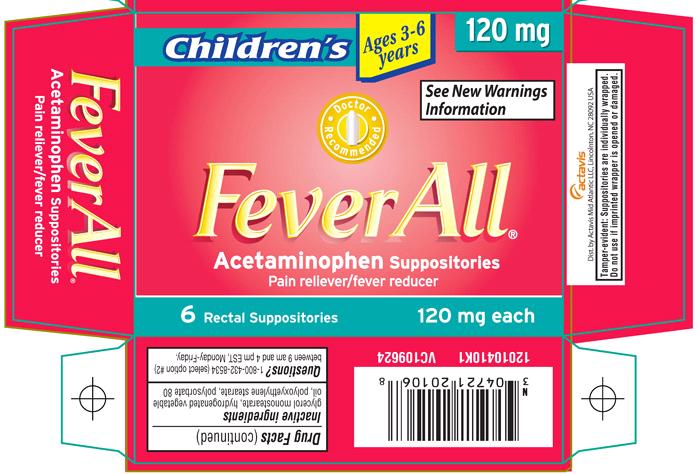
Children’s
Ages 3-6 years
120 mg
See New Warnings Information
FeverAll®
Acetaminophen Suppositories
Pain reliever/fever reducer
6 Rectal Suppositories
120 mg each

6 RECTAL SUPPOSITORIES CARTON LABEL
FeverAll®
Acetaminophen Suppositories
Pain reliever/fever reducer
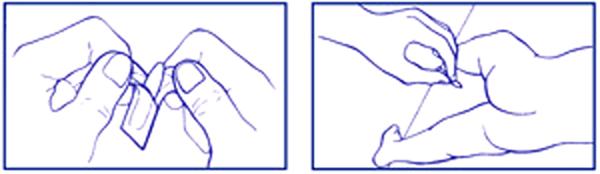
DIRECTIONS: When practical, cleanse area around rectum with mild soap and warm water and rinse thoroughly. Gently dry by patting or blotting with toilet tissue or a soft cloth before using FeverAll®.
Questions? 1-800-432-8534 (select option #2) between 9 am and 4 pm EST, Monday – Friday.
Distributed by:
Actavis Mid Atlantic LLC
1877 Kawai Road,
Lincolnton, NC 28092 USA
FORM NO. 2023/1/2
40-11600
Rev. 8/08
VC3350
|
FEVERALL CHILDREN
acetaminophen suppository | ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| ANDA | ANDA018337 | 04/01/2023 | |
| Labeler - Actavis Mid Atlantic LLC (809515898) |