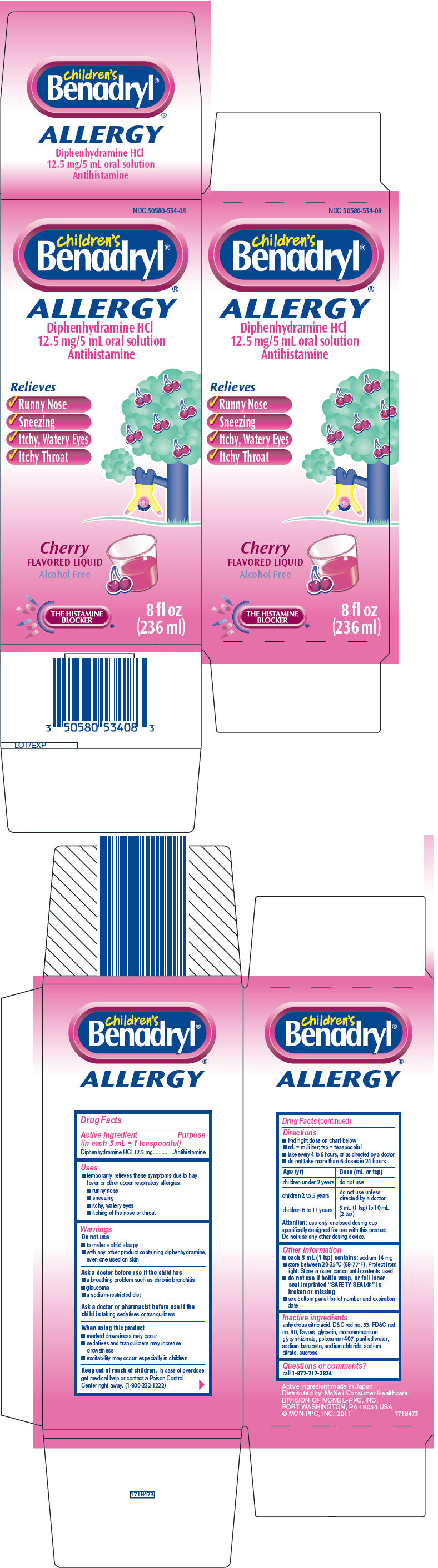
CHILDRENS BENADRYL ALLERGY
-
diphenhydramine hydrochloride solution
McNeil Consumer Healthcare Div. McNeil-PPC, Inc
Drug Facts
Diphenhydramine HCl 12.5 mg
Antihistamine
Ask a doctor or pharmacist before use if the child is taking sedatives or tranquilizers
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
| Age (yr) | Dose (mL or tsp) |
|---|---|
| children under 2 years | do not use |
| children 2 to 5 years | do not use unless directed by a doctor |
| children 6 to 11 years | 5 mL (1 tsp) to 10 mL (2 tsp) |
Attention: use only enclosed dosing cup specifically designed for use with this product. Do not use any other dosing device.
anhydrous citric acid, D&C red no. 33, FD&C red no. 40, flavors, glycerin, monoammonium glycyrrhizinate, poloxamer 407, purified water, sodium benzoate, sodium chloride, sodium citrate, sucrose
call 1-877-717-2824
NDC 50580-534-08
children's
Benadryl® ®
ALLERGY
Diphenhydramine HCl
12.5 mg/5 mL oral solution
Antihistamine
Relieves
Cherry
FLAVORED LIQUID
Alcohol Free
THE HISTAMINE
BLOCKER ®
8 fl oz
(236 ml)
|
CHILDRENS BENADRYL ALLERGY
diphenhydramine hydrochloride solution | ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC MONOGRAPH FINAL | part341 | 07/01/2023 | |
| Labeler - McNeil Consumer Healthcare Div. McNeil-PPC, Inc (878046358) |