ALCOHOL PREP
-
isopropyl alcohol swab
KYJ Medical Products Co.,Ltd.
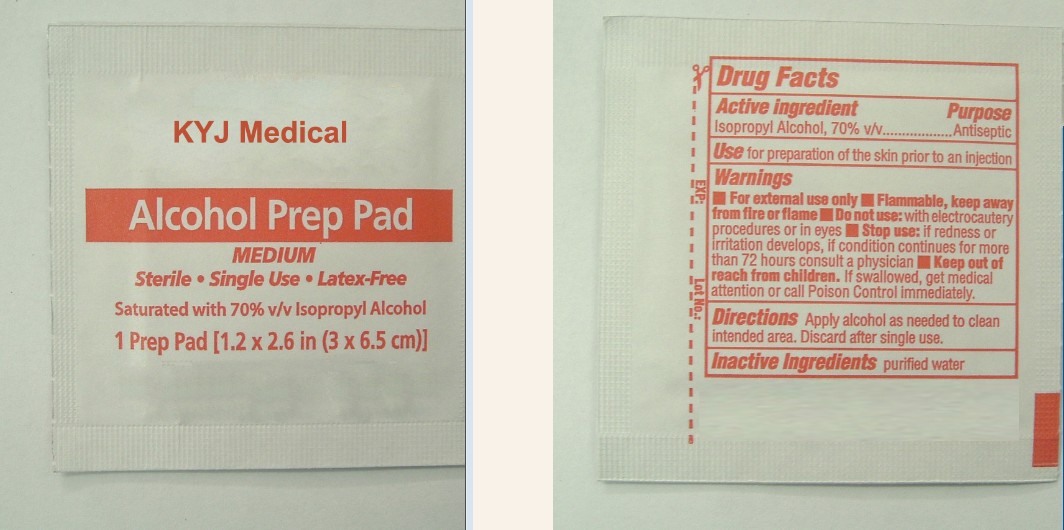
Use for preparation of the skin prior to an injection
For External use only.
Flammable,keep away from fire or flame.
Do not use with electrocautery procedures in eyes.
Stop use and ask a doctor if irritation and redness develop,condition persists for more than 72 hours .
Keep out of reach of children in case of accidental ingestion,seek professional assistance or consult a Poison Control Center immediately.
Apply alcohol as needed to clean intended area. Discard after single use.
Keep out of reach of children in case of accidental ingestion,seek professional assistance or consult a Poison Control Center immediately.
|
ALCOHOL PREP
isopropyl alcohol swab | ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part344 | 04/20/2011 | |
| Labeler - KYJ Medical Products Co.,Ltd. (421270350) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| KYJ Medical Products Co.,Ltd. | 421270350 | manufacture | |