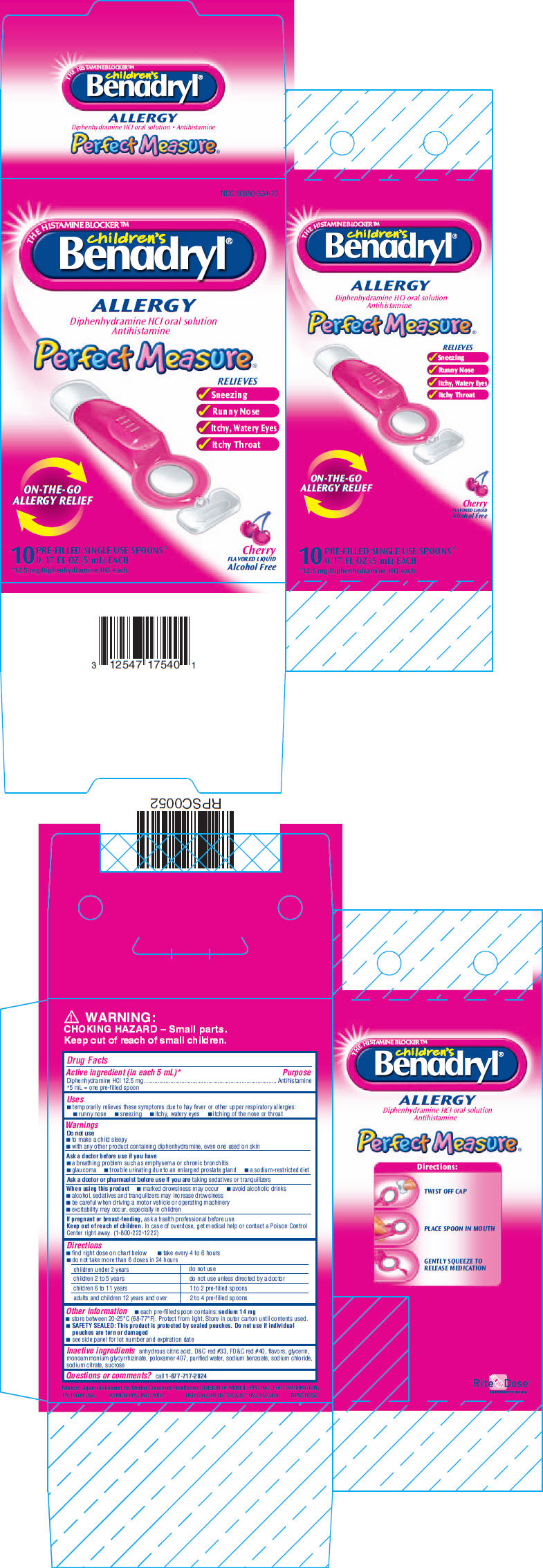
CHILDRENS BENADRYL ALLERGY
-
diphenhydramine hydrochloride liquid
McNeil Consumer Healthcare Div. McNeil-PPC, Inc
Drug Facts
Diphenhydramine HCl 12.5 mg
*5 mL = one pre-filled spoon
Antihistamine
Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
| children under 2 years | do not use |
| children 2 to 5 years | do not use unless directed by a doctor |
| children 6 to 11 years | 1 to 2 pre-filled spoons |
| adults and children 12 years and over | 2 to 4 pre-filled spoons |
anhydrous citric acid, D&C red #33, FD&C red #40, flavors, glycerin, monoammonium glycyrrhizinate, poloxamer 407, purified water, sodium benzoate, sodium chloride, sodium citrate, sucrose
call 1-877-717-2824
NDC 50580-334-10
THE HISTAMINE BLOCKER™
children's
Benadryl®
ALLERGY
Diphenhydramine HCI oral solution
Antihistamine
Perfect Measure®
RELIEVES
ON-THE-GO
ALLERGY RELIEF
10
PRE-FILLED SINGLE USE SPOONS*
0.17 FL OZ (5 mL) EACH
*12.5 mg Diphenhydramine HCl each
Cherry
FLAVORED LIQUID
Alcohol Free
|
CHILDRENS BENADRYL ALLERGY
diphenhydramine hydrochloride liquid | ||||||||||||||||||||||||||
| ||||||||||||||||||||||||||
| ||||||||||||||||||||||||||
| ||||||||||||||||||||||||||
| ||||||||||||||||||||||||||
| ||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC MONOGRAPH FINAL | part341 | 12/01/2023 | |
| Labeler - McNeil Consumer Healthcare Div. McNeil-PPC, Inc (878046358) |