CHILDRENS CHEWABLE ACETAMINOPHEN
-
acetaminophen tablet, chewable
Gemini Pharmaceuticals, Inc. dba Plus Pharma
Acetaminophen 80 mg
Pain Reliever/Fever Reducer
Temporarily relieves minor aches and pain due to:
■ The common cold ■ Flu ■ Headache ■ Sore throat
■ Toothache
Temporarily reduces fever.
Liver Warning: This Product contains acetaminophen. Severe liver damage may occur if your child takes ■ more than 5 doses in 24 hours, which is the maximum daily amount ■ with other drugs containing acetaminophen.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use ■ with any other other drug containing acetaminophen (prescription or non prescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or a pharmacist.
■ If your child is allergic to acetaminophen or any of the inactive ingredients in this products.
When using this product: Do not exceed recommended dose.
Stop use and ask a doctor if ■ Pain gets worse or lasts more than 5 days ■ Fever gets worse or lasts more than 3 days ■ New symptoms occur ■ Redness or swelling is present
These could be signs of a serious condition.
Keep out of reach of children.
Overdose Warning: Taking more than the recommended dose (overdose) may cause liver damage. In the case of overdose, get medical help or contact a poison Control Center right away. (1-800- 222-1222). Quick medical attention is critical even if you do not notice any signs or symptoms.
■ This products does not contain directions or complete warning for adult use.
■ Find right dose on the chart below. If possible, use weight to dose; otherwise, use age.
■ Chew before swallowing
■ If needed, repeat dose every 4 hours while symptoms lasts
■ Do not give more than 5 times in 24 hours
■ Do not give for more than 5 days unless directed by a doctor.
| WEIGHT | AGE | TABLETS |
| Under 24 lbs |
Under 2 years |
Ask a doctor |
| 24-35 lbs |
2-3 years |
2 tablets |
| 36-47 lbs |
4-5 years |
3 tablets |
| 48-59 lbs |
6-8 years |
4 tablets |
| 60-71 lbs |
9-10 years |
5 tablets |
| 72-95 lbs |
11 years |
6 tablets |
If you have any questions or comments, or to report an adverse drug event, please contact (800) 795-9775.
See New Warning Information
PlusPHARMATM
Children's Chewable
ACETAMINOPHEN
PAIN RELIEVER/FEVER REDUCER
CONTAINS NO ASPIRIN
*Compare the active ingredients to Children Chewable Tylenol®
30 Chewable Tablet ■ 80 mg Each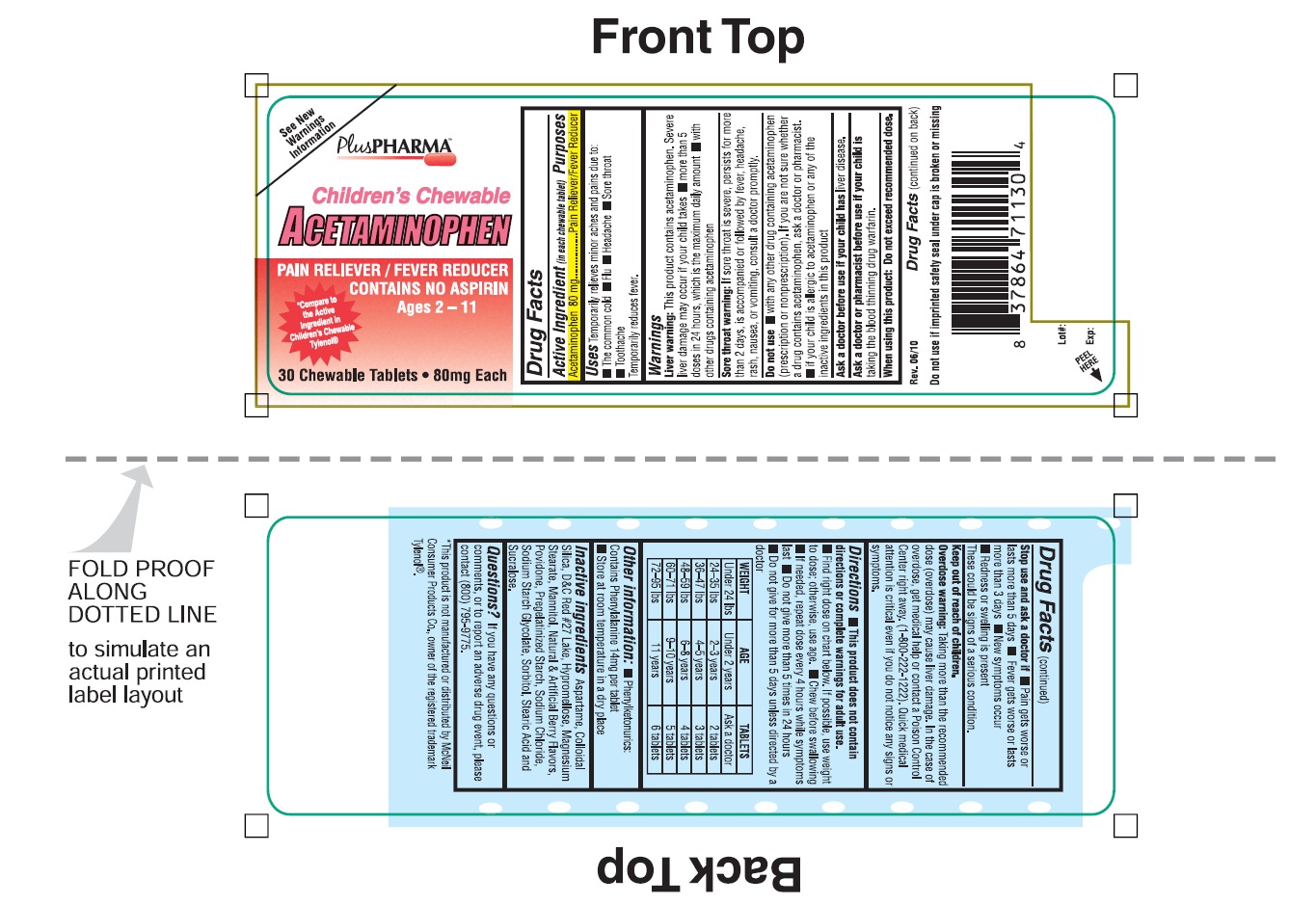
Rev. 06/10
Do not use if imprinted seal under cap is broken or missing.
|
CHILDRENS CHEWABLE ACETAMINOPHEN
acetaminophen tablet, chewable | ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part343 | 12/03/2023 | |
| Labeler - Gemini Pharmaceuticals, Inc. dba Plus Pharma (055942270) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Gemini Pharmaceuticals, Inc. dba Plus Pharma | 055942270 | manufacture | |