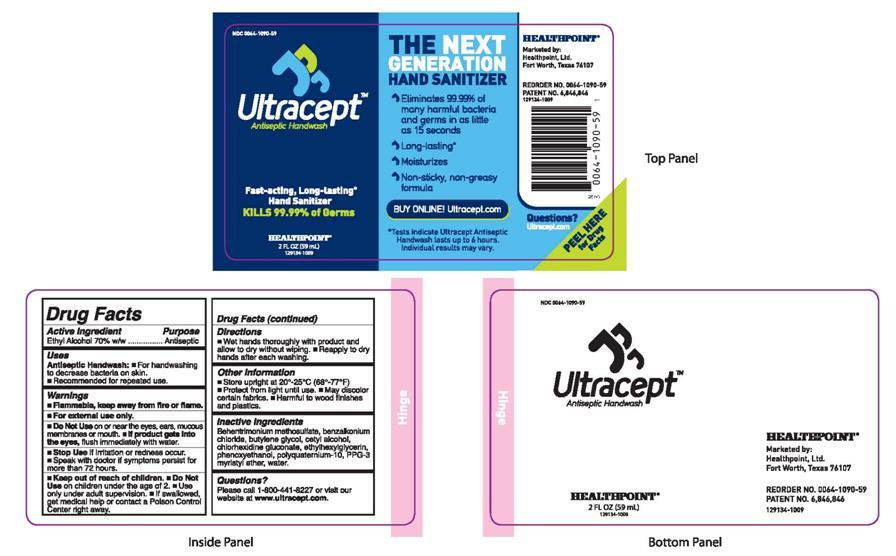
ULTRACEPT ANTISEPTIC HANDWASH
-
alcohol solution
Healthpoint, Ltd.
Drug Facts
Active Ingredient Purpose
Ethyl Alcohol 70% w/w Antiseptic
Uses
Antiseptic Handwash:
Directions
Other Information
Behentrimonium methosulfate, benzalkonium chloride, butylene glycol, cetyl alcohol, chlorhexidine gluconate, ethylhexylglycerin, phenoxyethanol, polyquaternium-10, PPG-3 myristyl ether, water.
Please call 1-800-441-8227 or visit our website at www.ultracept.com.
PRINCIPAL DISPLAY PANEL
NDC 0064-1090-59
ULTRACEPTTM Antiseptic Handwash
Fast-acting, Long-Lasting* Hand Sanitizer
Kills 99.99% of Germs
THE NEXT GENERATION HAND SANITIZER
BUY ONLINE! Ultracept.com
*Tests indicate Ultracept Antiseptic Handwash lasts up to 6 hours. Individual results may vary.
HEALTHPOINT®
Marketed by:
Healthpoint, Ltd.
Fort Worth, Texas 76107
REORDER NO. 0064-1090-59
PATENT NO. 6,846,846
129134-1009
Questions?
Ultracept.com
|
ULTRACEPT
ANTISEPTIC HANDWASH ethyl alcohol solution | ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part333 | 11/09/2022 | |
| Labeler - Healthpoint, Ltd. (965634504) |