GUAIFENESIN DM
-
guaifenesin and
dextromethorphan solution
Pharmaceutical Associates, Inc.
Drug Facts
| Active ingredients (in each 5 mL teaspoonful) |
Purposes |
| Guaifenesin 200 mg | Expectorant |
| Dextromethorphan HBr 10 mg | Cough Suppressant |
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Stop use and ask a doctor if cough lasts more than 7 days, comes back, or is accompanied by fever, rash, or persistent headache. These could be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
| age | dose |
|---|---|
| adults and children 12 years and over | 10 mL (2 teaspoonsful) every 4 hours |
| children 6 to under 12 years of age | 5 mL (1 teaspoonful) every 4 hours |
| children 2 to under 6 years of age | 2.5 mL (1/2 teaspoonful) every 4 hours |
| children under 2 years | consult a doctor |
Acesulfame K, citric acid, FD&C Red No. 40, flavoring, glycerin, menthol, polyethylene glycol, propylene glycol, purified water, sodium benzoate, sodium citrate, sodium saccharin, sorbitol and sucralose.
Call 1-800-845-8210. You may also report serious side effects to this phone number.
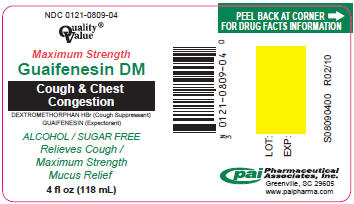
NDC 0121-0809-04
Quality®
Value
Maximum Strength
Guaifenesin DM
Cough & Chest
Congestion
DEXTROMETHORPHAN HBr (Cough Suppressant)
GUAIFENESIN (Expectorant)
ALCOHOL / SUGAR FREE
Relieves Cough /
Maximum Strength
Mucus Relief
4 fl oz (118 mL)
Delivers 5 mL
NDC 0121-4809-05
MAXIMUM STRENGTH
GUAIFENESIN DM
Guaifenesin (Expectorant)
Dextromethorphan HBr (Cough Suppressant)
200 mg/10 mg per 5 mL
Alcohol Free / Sugar Free
FOR INSTITUTIONAL USE ONLY
PHARMACEUTICAL ASSOCIATES, INC.
GREENVILLE, SC 29605
SEE INSERT

|
GUAIFENESIN DM
guaifenesin and dextromethorphan solution | ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part341 | 05/17/2010 | |
|
GUAIFENESIN DM
guaifenesin and dextromethorphan solution | ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part341 | 05/17/2010 | |
| Labeler - Pharmaceutical Associates, Inc. (044940096) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Pharmaceutical Associates, Inc. | 044940096 | MANUFACTURE | |