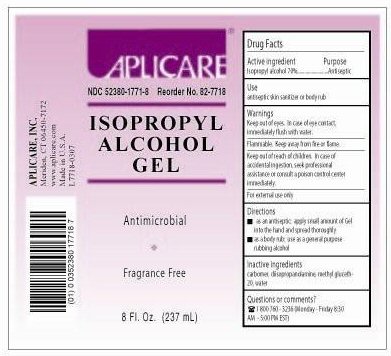
APLICARE ISOPROPYL ALCOHOL
-
isopropyl alcohol gel
Aplicare, Inc.
Isopropyl Alcohol 70%
Antiseptic
Keep out of eyes. If contact with eyes occurs, rinse promptly and throughly with water.
Flammable. Keep away from fire or flame.
For external use only.
Keep out of reach of children. In case of accidental
ingestion, seek professional assistance
or consult a poison control center immediately.
Questions or comments?
1-800-760-3236 (Mon to Fri 8:30 AM - 5:00 PM EST)
Aplicare Isopropyl Alcohol Gel 8 oz
|
APLICARE ISOPROPYL ALCOHOL
isopropyl alcohol gel | ||||||||||||||||||||
| ||||||||||||||||||||
| ||||||||||||||||||||
| ||||||||||||||||||||
| ||||||||||||||||||||
| ||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part333 | 09/01/2023 | |
| Labeler - Aplicare, Inc. (107255002) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Aplicare, Inc. | 058377631 | manufacture | |