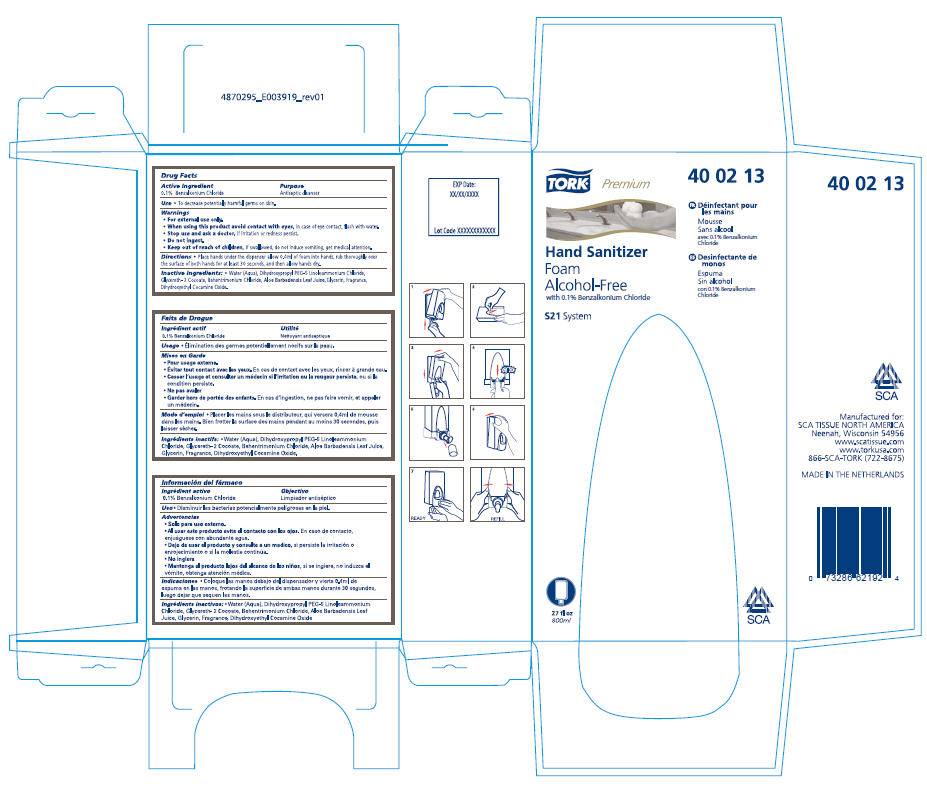
TORK PREMIUM HAND SANITIZER FOAM ALCOHOL-FREE
-
benzalkonium chloride liquid
SCA Tissue North America
Drug Facts
0.1% Benzalkonium Chloride
Antiseptic cleanser
Manufactured for:
SCA TISSUE NORTH AMERICA
Neenah, Wisconsin 54956
www.scatissue.com
www.torkusa.com
866-SCA-TORK (722-8675)
TORK®
Premium
40 02 13
Hand Sanitizer
Foam
Alcohol-Free
with 0.1% Benzalkonium Chloride
S21 System
FR Déinfectant pour
les mains
Mousse
Sans alcool
avec 0.1% Benzalkonium
Chloride
ES Desinfectante de
monos
Espuma
Sin alcohol
con 0.1% Benzalkonium
Chloride
27 fl oz
800ml
SCA
|
TORK PREMIUM HAND SANITIZER FOAM ALCOHOL-FREE
benzalkonium chloride liquid | ||||||||||||||||||||||||
| ||||||||||||||||||||||||
| ||||||||||||||||||||||||
| ||||||||||||||||||||||||
| ||||||||||||||||||||||||
| ||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part333 | 03/03/2023 | |
| Labeler - SCA Tissue North America (005694349) |
| Registrant - Rubbermaid Commercial Products LLC (049924368) |