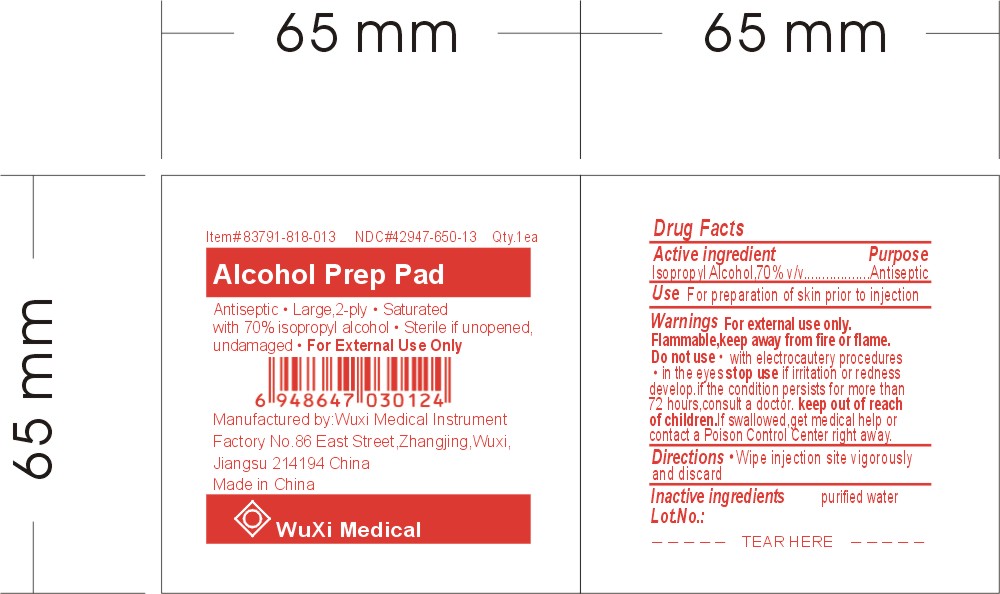
ALCOHOL PREP PAD
-
isopropyl alcohol swab
Wuxi Medical Instrument Factory
Item# 83791-818-013 NDC# 42947-650-13 Qty. 1ea
Alcohol Prep Pad
Antiseptic * Large, 2-ply * Saturated with 70% isopropyl alcohol
Sterile if unopened, undamaged
For External Use Only
Manufactured by:
Wuxi Medical Instrument Factory
No.86 East Street, Zhangjing, Wuxi, Jiangsu 214194 China
Made in China
Active Ingredient
Isopropyl Alcohol, 70% v/v
Purpose
Antiseptic
Use
For preparation of skin prior to injection
Do not use
* with electrocautery procedures
* in the eyes
Stop use
if irritation or redness develop.
if the condition persists for more than 71 hours, consult a doctor.
Keep out of reach of children.
If swallowed, get medical help or contact Poison Control Center right away.
Directions
Wipe injection site vigorously and discard
Inactive ingredient
purified water
Image of Pouch Label
|
ALCOHOL PREP PAD
isopropyl alcohol swab | ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part333 | 01/01/2023 | |
| Labeler - Wuxi Medical Instrument Factory (421292863) |
| Registrant - Wuxi Medical Instrument Factory (421292863) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Wuxi Medical Instrument Factory | 421292863 | manufacture | |