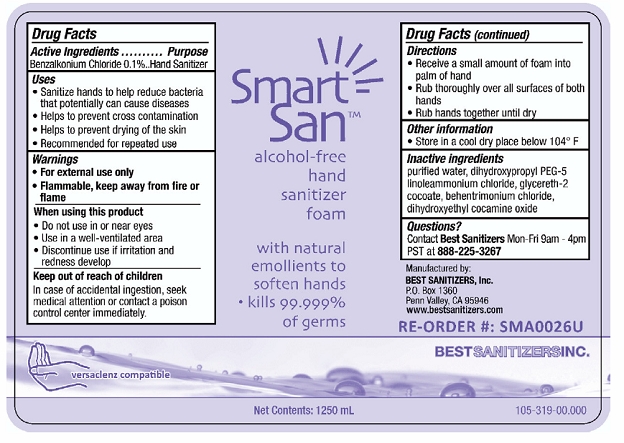
SMART SAN ALCOHOL FREE HAND SANITIZER FOAM
-
benzalkonium chloride liquid
Best Sanitizers, Inc
Benzalkonium Chloride 0.1%
Hand Sanitizer
In case of accidental ingestion, seek medical attention or contact a poison control center immediately.
Store in a cool dry place below 104° F
purified water, dihydroxypropyl PEG-5, linoleammonium chloride, glycereth-2 cocoate, behentrimonium chloride, dihydroxyethyl cocamine oxide
Contact Best Sanitizers Mon-Fri 9am-4pm PST at 888-225-3267
NDC 59900-118-06
NDC 59900-118-12
NDC 59900-118-48
Smart San™ alcohol-free hand sanitizer foam
|
SMART SAN ALCOHOL FREE HAND SANITIZER FOAM
benzalkonium chloride liquid | ||||||||||||||||||||
| ||||||||||||||||||||
| ||||||||||||||||||||
| ||||||||||||||||||||
| ||||||||||||||||||||
| ||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part352 | 07/15/2009 | |
| Labeler - Best Sanitizers, Inc (957473614) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Best Sanitizers, Inc | 627278224 | manufacture | |