ALCOHOL PREP PAD
-
isopropyl alcohol swab
Target Corporation
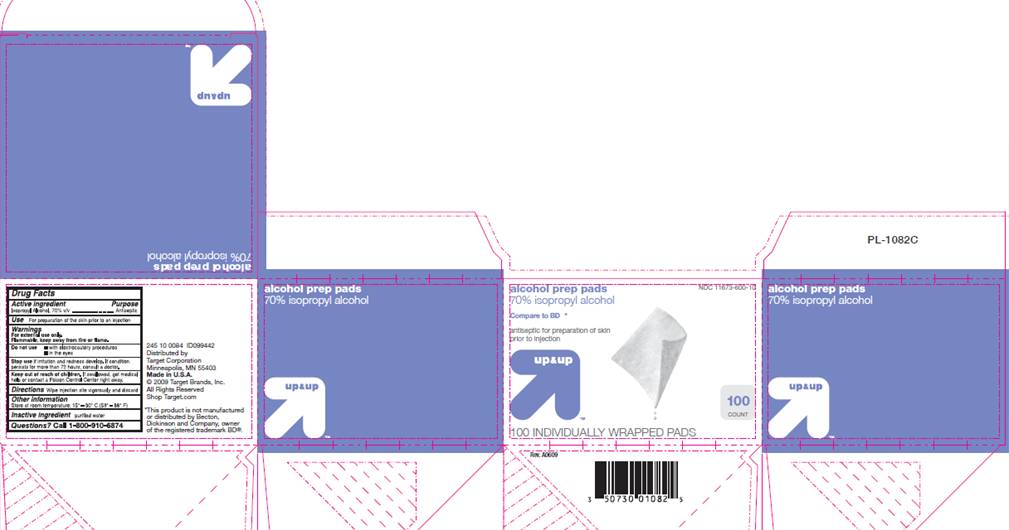
DRUG FACTSACTIVE INGREDIENT
Isopropyl Alcohol 70% v/v
PURPOSE
Antiseptic
USE
For preparation of the skin prior to an injection
WARNINGS
For external use only.
Flammable, keep away from fire or flame.Do not use
- with electrocautery procedures
- in the eyes
Stop use
if irritation and redness develop. If condition persists for more than 72 hours, consult a doctor.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
DIRECTIONS
Wipe injection site vigorously and discard.
OTHER INFORMATION
Store at room temperature 15°- 30° C (59° - 86° F)
INACTIVE INGREDIENT
purified water
Questions?
Call 1-800-910-6874
PACKAGE INFORMATION
up and up
NDC 11673-600-10
alcohol prep pads70% isopropyl alcohol
Compare to BD®*
antiseptic for preparation of skin prior to injection
100 count
100 INDIVIDUALLY WRAPPED PADS
Distributed by Target Corporation
Minneapolis, MN 55403
Made in U.S.A.
© 2009 Target Brands, Inc.
All Rights Reserved
Shop Target.com
*This product is not manufactured or distributed by Becton Dickinson and Company, owner of the registered trademark BD®.
ALCOHOL PREP PAD
isopropyl alcohol
swab
|
|
|
|
|
|
Revised: 02/2010Target Corporation