ISOPROPYL ALCOHOL
-
isopropyl alcohol swab
LernaPharm Loris Inc.
Isopropyl Alcohol 70% v/v
Antiseptic
Flammable, keep away from fire or flame. For external use only.
Do not use in the eyes ● apply over large areas of the body
Tear open package and rub skin briskly in circular motion from injection site outward.
Store at room temperature between 59 - 86 0F (15 - 30 0C)
NDC 68356-117-02 PRODUCT # 117-02U
LORIS - ALCOHOL SWABS
MEDIUM
Saturated with 70% v/v isopropyl alcohol, USP
Single use only ● For external use only ● Easy to use ● Economical
200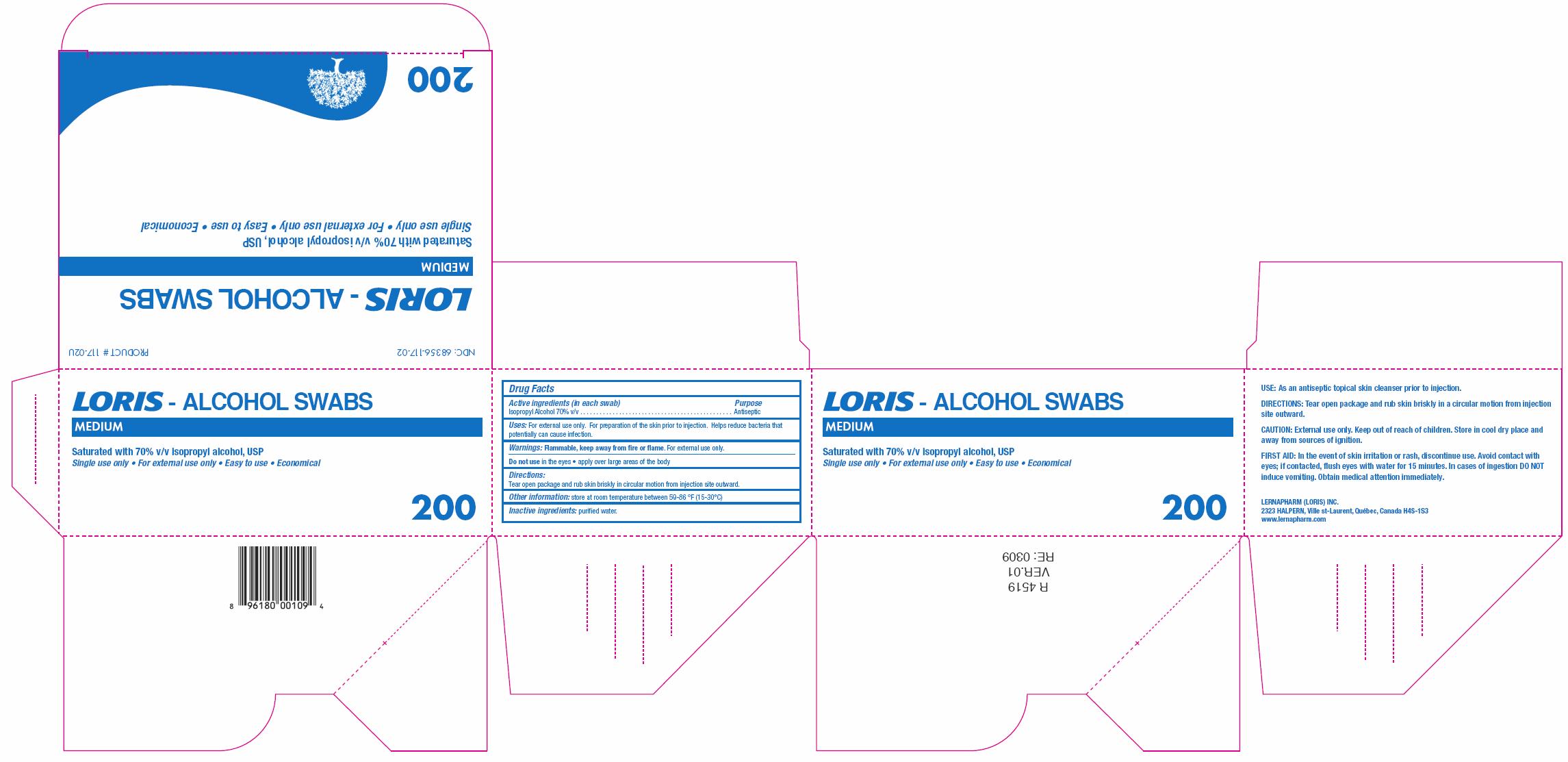
|
ISOPROPYL ALCOHOL
isopropyl alcohol swab | ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part333 | 01/04/2023 | |
| Labeler - LernaPharm Loris Inc. (206940905) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LernaPharm Loris Inc. | 206940905 | manufacture | |