APLICARE ISOPROPYL ALCOHOL
-
isopropyl alcohol solution
Aplicare, Inc.
One Ounce 70% Isopropyl Alcohol
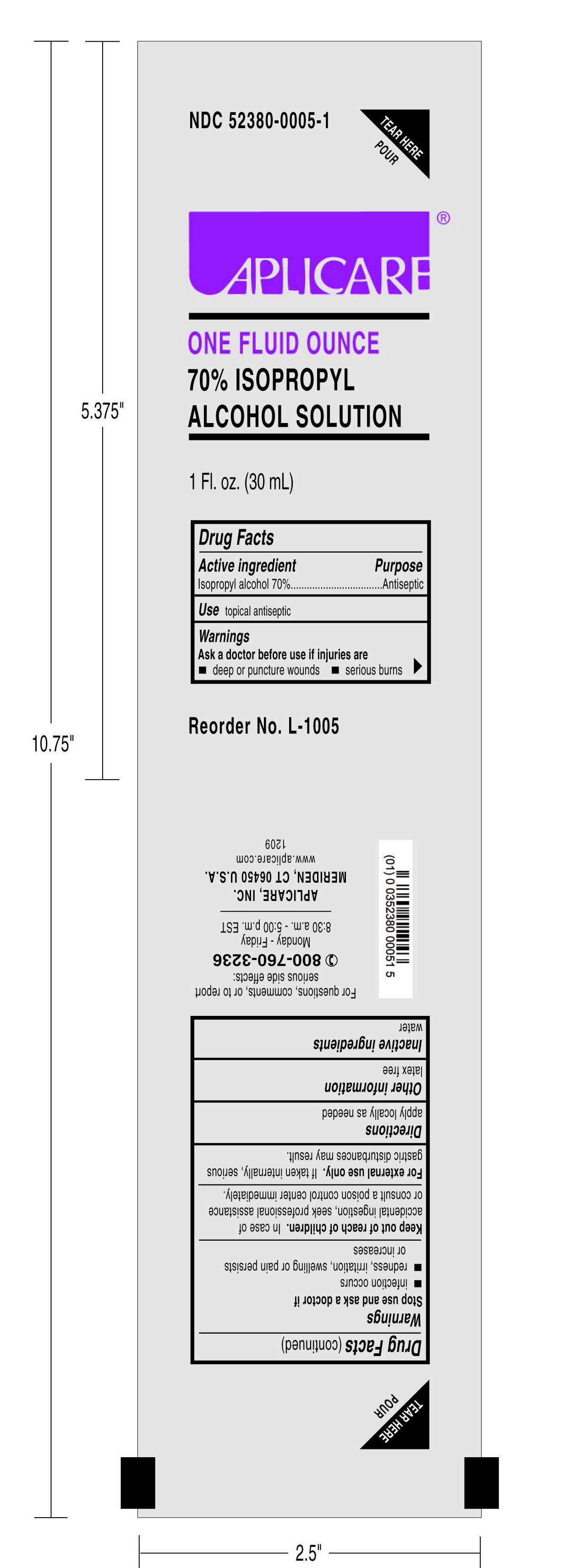
70% Isopropyl Alcohol
Antiseptic
Ask a doctor before use if injuries areStop use
and ask a doctor if
Keep out of reach of children. In case of accidental ingestion, seek professionalassistance or consult a poison control center immediately.
For external use only. If taken internally, serious gastric disturbances may result.
|
APLICARE ISOPROPYL ALCOHOL
isopropyl alcohol solution | ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part333 | 01/01/2023 | |
| Labeler - Aplicare, Inc. (107255002) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Aplicare, Inc. | 107255002 | manufacture | |