ISOPROPYL ALCOHOL PREP PAD
-
isopropyl alcohol swab
H and P Industries, Inc. dba Triad Group
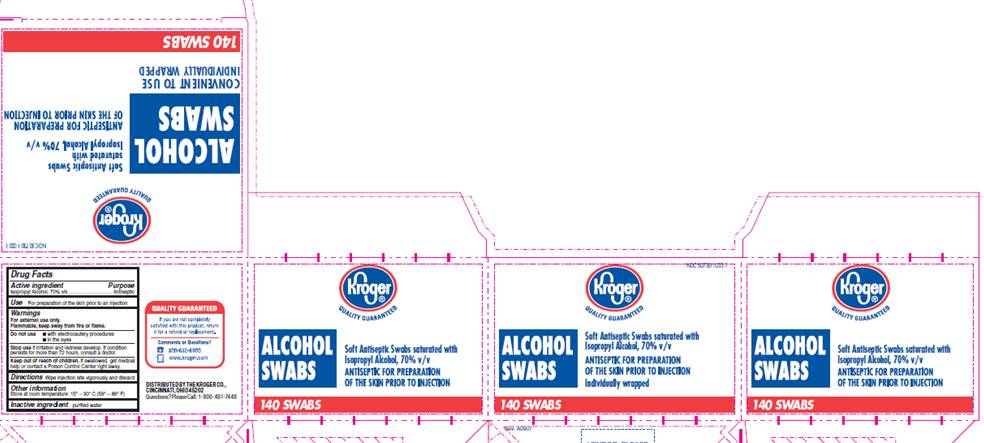
DRUG FACTSACTIVE INGREDIENT
Isopropyl Alcohol 70% v/v
PURPOSE
Antiseptic
USE
For preparation of the skin prior to an injection
WARNINGS
For external use only.
Flammable, keep away from fire or flame.Do not use
- with electrocautery procedures
- in the eyes
Stop use
if irritation and redness develop. If condition persists for more than 72 hours, consult a doctor.
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
DIRECTIONS
Wipe injection site vigorously and discard
OTHER INFORMATION
Store at room temperature 15° - 30° C (59° - 86° F)
INACTIVE INGREDIENT
purified water
PACKAGE INFORMATION
NDC 50730-1033-1
Kroeger®
Quality Guaranteed
ALCOHOL SWABSSoft Antiseptic Swabs saturated with
Isopropyl Alcohol, 70% v/v
ANTISEPTIC FOR PREPARATION OF THE SKIN PRIOR TO INJECTION
Individually wrapped
140 SWABS
Quality Guaranteed
If you are not completely satisfied with this product, return it for a refund or replacement.
Comments or Questions?
800-632-6900
www.kroeger.com
DISTRIBUTED BY THE KROEGER CO.,
CINCINNATI, OHIO 45202
Questions? Please Call: 1-800-491-7448
ISOPROPYL ALCOHOL PREP PAD
isopropyl alcohol
swab
|
|
|
|
|
|
Revised: 12/2009H and P Industries, Inc. dba Triad Group