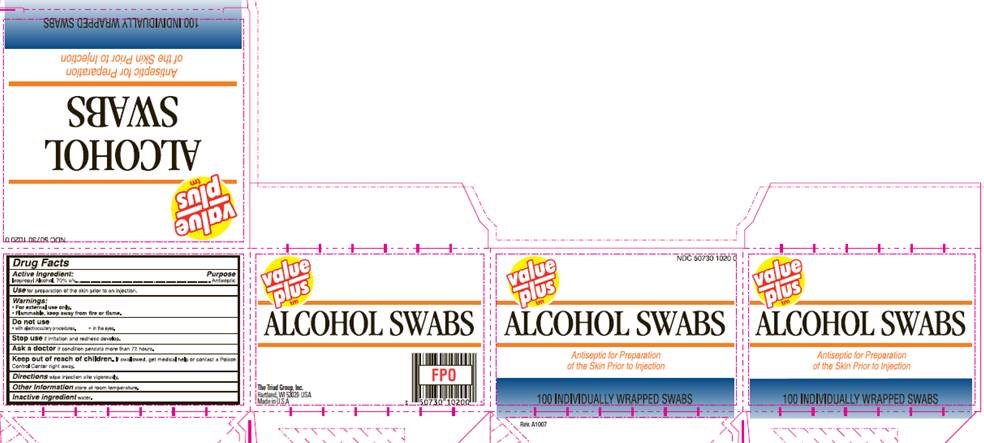
ISOPROPYL ALCOHOL SWABS
-
isopropyl alcohol swab
H and P Industries, Inc. dba Triad Group
Isopropyl Alcohol 70% v/v
Antiseptic
If swallowed, get medical help or contact a Poison Control Center right away.
NDC 50730-1020-0
value plus
ALCOHOL SWABS
Antiseptic for Preparation
of the Skin Prior to Injection
|
ISOPROPYL ALCOHOL SWABS
isopropyl alcohol swab | ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part333 | 10/01/2023 | |
| Labeler - H and P Industries, Inc. dba Triad Group (050259597) |