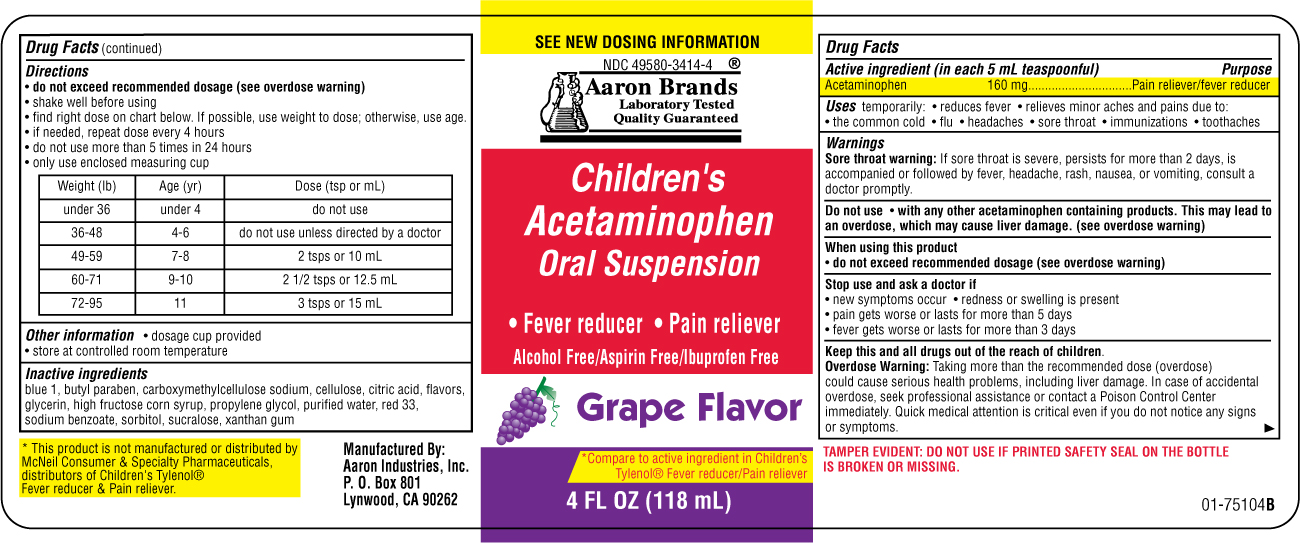
CHILDRENS ACETAMINOPHEN ORAL SUSPENSION GRAPE FLAVOR
-
acetaminophen suspension
Aaron Industries, Inc.
(in each 5 mL, 1 teaspoon)
Acetaminophen 160 mg
Acetaminophen
160 mg.............................Pain reliever/fever reducer
temporarily reduces fever
temporarily relieves minor aches and pains due to:
• the common cold • flu • headache
• sore throat • toothache
Liver warning: This product contains acetaminophen.
Severe liver damage may occur if your child takes:
• more than 5 doses in 24 hours, which is the
maximum daily amount
• with other drugs containing acetaminophen
Sore throat warning: If sore throat is severe, persists
for more than 2 days, is accompanied or followed by
fever, headache, rash, nausea, or vomiting, consult a
doctor promptly.
with any other drug containing
acetaminophen (prescription or non-prescription).
If you are not sure whether a drug contains
acetaminophen, ask a doctor or pharmacist
liver disease.
is taking the blood thinning drug warfarin.
do not exceed recommended dose
(see overdose warning)
• new symptoms occur
• redness or swelling is present
• pain gets worse or lasts more than 5 days
• fever gets worse or lasts more than 3 days
These could be signs of a serious condition.
Enter section text here
Taking more than the
recommended dose (overdose) may cause liver
damage. In case of accidental overdose, seek
professional assistance or contact a Poison Control
Center immediately. Quick medical attention is
critical even if you do not notice any signs or
symptoms.
this product does not contain directions or
complete warnings for adult use.
• shake well before using
• find right dose on chart below. If possible, use
weight to dose; otherwise, use age.
• use only enclosed dosing cup designed for use
with this product. Do not use any other dosing
device.
• if needed, repeat dose every 4 hours while
symptoms last
• do not give more than 5 times in 24 hours
• do not give more than 5 days unless directed
by a doctor
Weight (lb) Age (yr) Dose (tsp or mL)
under 24
under 2 ask a doctor
24-35
2-3 1 teaspoon or 5 mL
36-47
4-5 1 1/2 teaspoons or 7.5 mL
48-59
6-8 2 teaspoons or 10 mL
60-71
9-10 2 1/2 teaspoons or 12.5 mL
72-95
11 3 teaspoons or 15 mL
• store at controlled room temperature
• see bottom panel for lot number and
expiration date
blue 1, butyl paraben, carboxymethylcellulose
sodium, cellulose, citric acid, flavors, glycerin,
high fructose corn syrup, propylene glycol, purified
water, red 33, sodium benzoate, sorbitol, sucralose,
xanthan gum
Aaron Brands
Childrens Acetaminophen Oral Suspension
• Fever reducerGrape Flavor
Compare to active ingredient in Childrens Tylenol Fever reducer Pain reliever
4 FL OZ (118 mL)
|
CHILDRENS ACETAMINOPHEN ORAL SUSPENSION
GRAPE FLAVOR acetaminophen suspension | ||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part341 | 07/29/2009 | |
| Labeler - Aaron Industries, Inc. (101896231) |
| Registrant - Aaron Industries, Inc. (101896231) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Aaron Industries, Inc. | 101896231 | analysis, manufacture | |