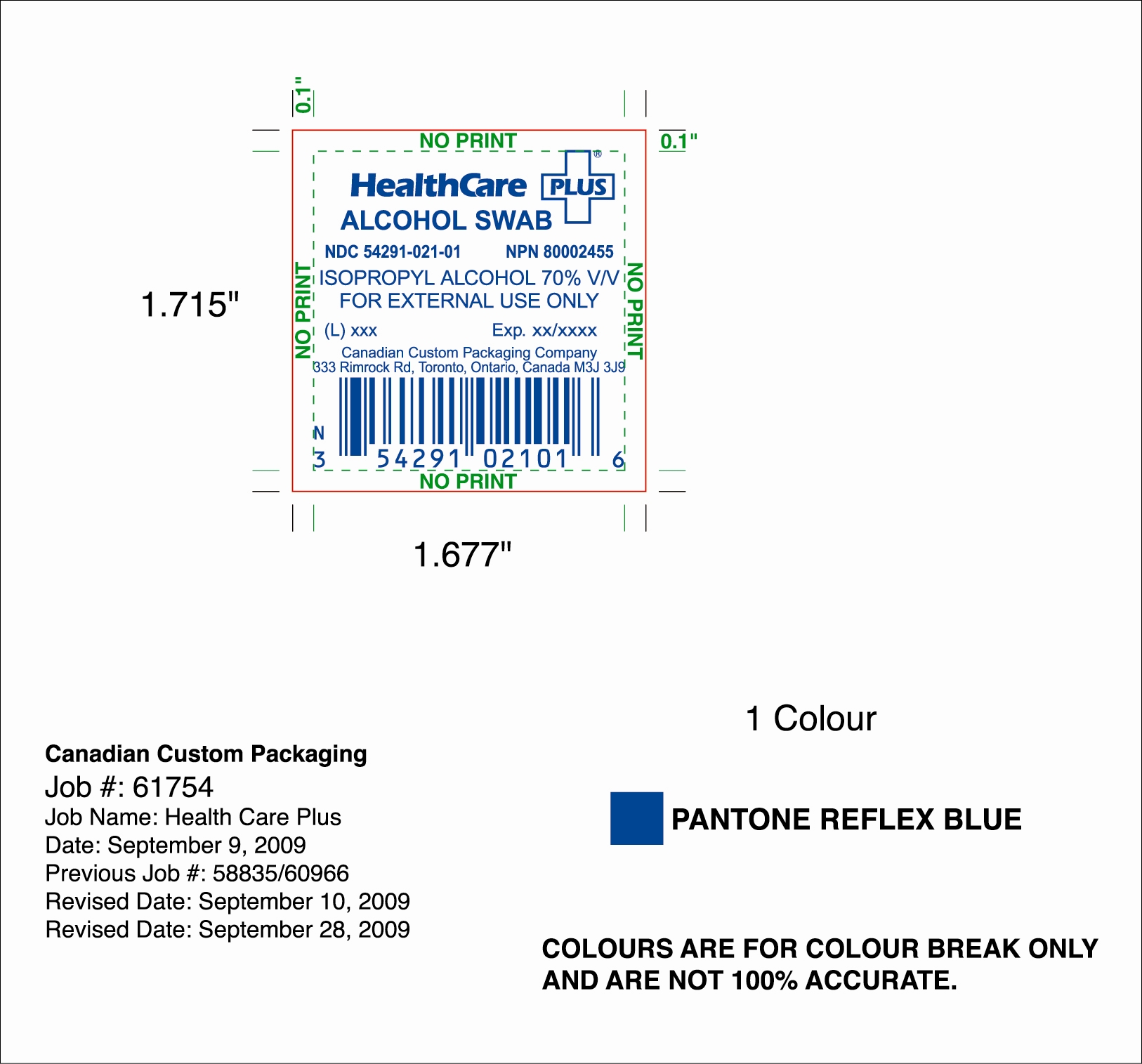
HEALTHCARE PLUS
-
isopropyl alcohol swab
Canadian Custom Packaging Company
Isopropyl Alcohol 70% v/v
Antiseptic
For antiseptic skin cleansing prior to injection and to help decrease the risk of infection in minor cuts and scrapes.
if irritation and redness develop. If condition persists for more than 72 hours, consult a doctor.
If swallowed, get medical help or contact a Poison Control Center right away.
Rub skin briskly in a circular motion from injection site outward.
Store at room temperature: 15o - 30o C (59o - 86o F)
purified water

|
HEALTHCARE
PLUS alcohol swabs swab | ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part333 | 10/05/2023 | |
| Labeler - Canadian Custom Packaging Company (207062514) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Canadian Custom Packaging Company | 207062514 | manufacture | |