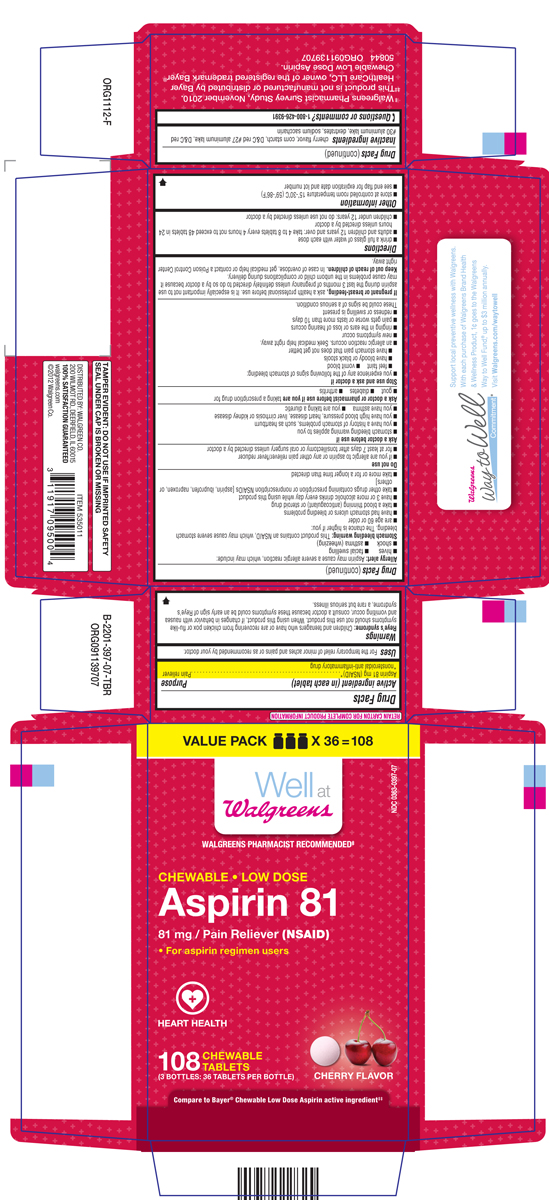
LOW DOSE ASPIRIN
-
aspirin tablet, chewable
WALGREEN CO.
Aspirin 81 mg (NSAID)*
*nonsteroidal anti-inflammatory drug
Pain reliever
For the temporary relief of minor aches and pains or as recommended by your doctor.
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrom, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction, which may include:
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
taking a prescription drug for
These could be signs of a serious condition.
ask a health professional before use. It is especially important not to use aspiring during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
In case of overdose, get medical help or contact a Poison Control Center right away.
cherry flavor, corn starch, D&C red #27 aluminum lake, D&C red #30 aluminum lake, dextrates, sodium saccharin
VALUE PACK (3 BOTTLES) x 36 = 108
Well at Walgreens
WALGREENS PHARMACIST RECOMMENDEDǂ
NDC 0363-0397-07
CHEWABLE • LOW DOSE
Aspirin 81
81 mg / Pain Reliever (NSAID)
• For aspirin regimen users
HEART HEALTH
108 CHEWABLE TABLETS
(3 BOTTLES: 36 TABLETS PER BOTTLE CHERRY FLAVOR
Compare to Bayer® Chewable Aspirin active ingredientǂǂ
ǂ Walgreens Pharmacist Survey Study, November 2010
ǂǂThis product is not manufactured or distributed by Bayer Healthcare LLC, owner of the registered trademark Bayer® Chewable Aspirin.
50844 ORG091139707
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
Walgreens 44-397
|
LOW DOSE ASPIRIN
aspirin tablet, chewable | ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC MONOGRAPH NOT FINAL | part343 | 09/04/2023 | |
| Labeler - WALGREEN CO. (008965063) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 038154464 | PACK | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 832867894 | MANUFACTURE | |