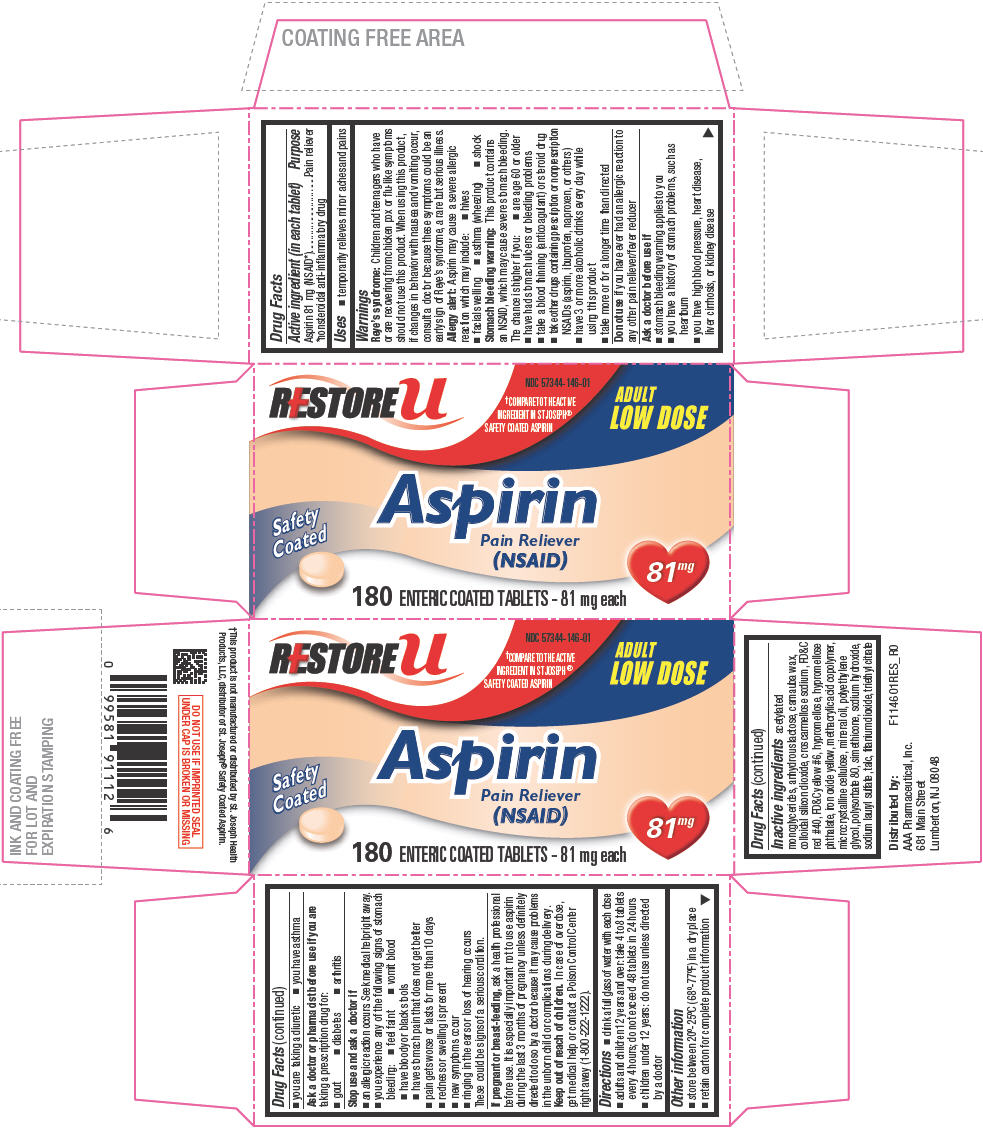
ASPIRIN
-
aspirin tablet, delayed release
AAA Pharmaceutical, Inc.
Drug Facts
Aspirin 81 mg (NSAID1)
Pain reliever
Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Aspirin may cause a severe allergic reaction which may include:
This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
Do not use if you have ever had an allergic reaction to any other pain reliever/fever reducer
Ask a doctor or pharmacist before use if you are taking a prescription drug for:
These could be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use. It is especially important not to use aspirin during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222).
acetylated monoglycerides, anhydrous lactose, carnauba wax, colloidal silicon dioxide, croscarmellose sodium, FD&C red #40, FD&C yellow #6, hypromellose, hypromellose phthalate, iron oxide yellow, methacrylic acid copolymer, microcrystalline cellulose, mineral oil, polyethylene glycol, polysorbate 80, simethicone, sodium hydroxide, sodium lauryl sulfate, talc, titanium dioxide, triethyl citrate
Distributed by:
AAA Pharmaceutical, Inc.
681 Main Street
Lumberton, NJ 08048
RESTORE u
NDC 57344-146-01
†COMPARE TO THE ACTIVE
INGREDIENT IN ST JOSEPH®
SAFETY COATED ASPIRIN
ADULT
LOW DOSE
Safety
Coated
Aspirin
Pain Reliever
(NSAID)
81mg
180 ENTERIC COATED TABLETS - 81 mg each
|
ASPIRIN
aspirin tablet, delayed release | ||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC MONOGRAPH NOT FINAL | part343 | 12/15/2012 | |
| Labeler - AAA Pharmaceutical, Inc. (181192162) |