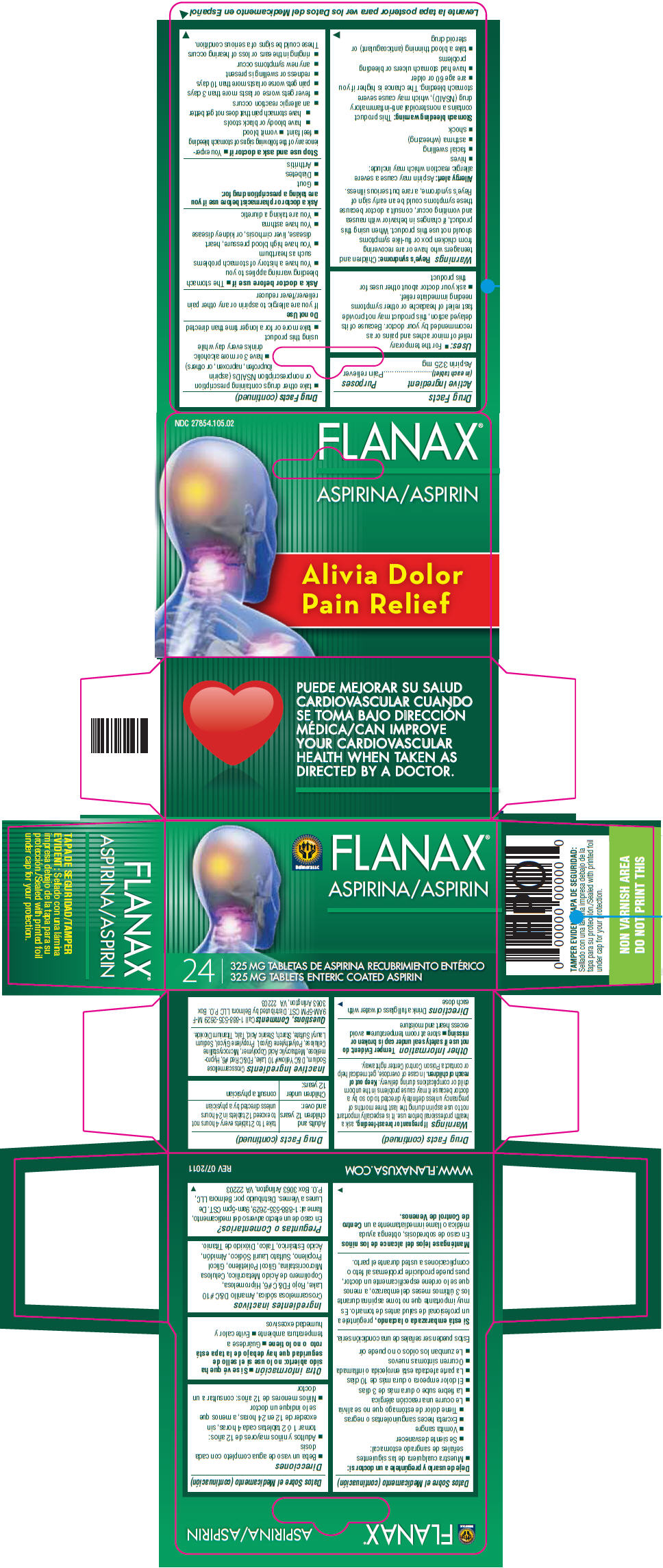
FLANAX ASPIRIN PAIN RELIEVER
-
aspirin tablet
Belmora LLC
Drug Facts
Aspirin 325 mg
Pain reliever
Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Aspirin may cause a severe allergic reaction which may include:
This product contains a nonsteroidal anti-inflammatory drug (NSAID), which may cause severe stomach bleeding. The chance is higher if you
If you are allergic to aspirin or any other pain reliever/fever reducer
Ask a doctor or pharmacist before use if you are taking a prescription drug for:
These could be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use. It is especially important not to use aspirin during the last three months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Temper Evident: do not use if safety seal under cap is broken or missing
Drink a full glass of water with each dose
| Adults and children 12 years and over: | take 1 to 2 tablets every 4 hours not to exceed 12 tablets in 24 hours unless directed by a physician |
| Children under 12 years: | consult a physician |
Crosscarmellose Sodium, D&C Yellow# 10 Lake, FD&C Red #6, Hypromellose, Methacrylic Acid Copolymer, Microcrystalline Cellulose, Polyethylene Glycol, Propylene Glycol, Sodium Lauryl Sulfate, Starch, Stearic Acid, Talc, Titanium Dioxide.
Call 1-888-535-2629 M-F 9AM-5PM CST. Distributed by Belmora LLC P.O. Box 3063 Arlington, VA 22203
Belmora LLC
FLANAX®
ASPIRIN
24
325 MG TABLETS ENTERIC COATED ASPIRIN
|
FLANAX ASPIRIN PAIN RELIEVER
aspirin tablet | ||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC MONOGRAPH FINAL | part343 | 12/06/2023 | |
| Labeler - Belmora LLC (112753244) |
| Registrant - Pharbest Pharmaceuticals Corporation (557054835) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Belmora LLC | 112753244 | LABEL | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Pharbest Pharmaceuticals Corporation | 557054835 | MANUFACTURE | |