ASPIRIN
-
aspirin tablet
Rugby Laboratories Inc.
(in each tablet)
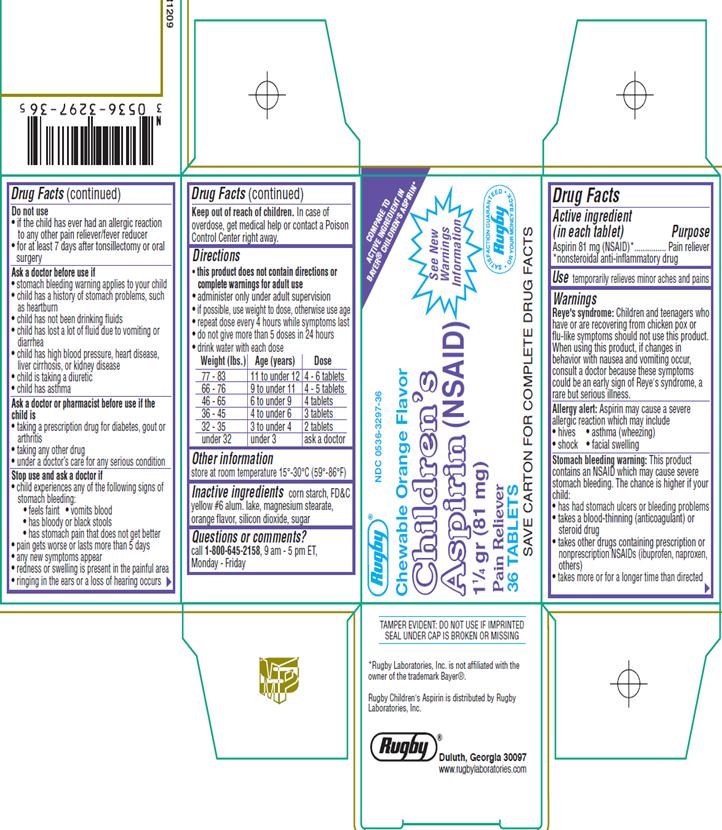
Aspirin 81 mg (NSAID)
*nonsteroidal anti-inflammatory drug
Pain Reliever
Reye’s Syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early signs of Reye’s syndrome, a rare but serious illness.
Allergy Alert: Aspirin may cause a severe allergic reaction which may include
Stomach Bleeding Warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if your child
Do not use
Ask a doctor before use if
Ask a doctor or pharmacist before use if the child is
Stop use and ask a doctor if
Keep out of reach of children. In case of overdose, get medical help or contact a poison control center right away.
| Weight (lbs.) | Age (years) | Dose |
| 77 – 83 | 11 to under 12 | 4 – 6 tablets |
| 66 – 76 | 9 to under 11 | 4 - 5 tablets |
| 46 – 65 | 6 to under 9 | 4 tablets |
| 36 – 45 | 4 to under 6 | 3 tablets |
| 32 – 35 | 3 to under 4 | 2 tablets |
| under 32 | under 3 | ask a doctor |
store at room temperature 15°-30°C (59°-86°F)
corn starch, FD&C Yellow #6 alum. lake, magnesium stearate, orange flavor, silicon dioxide, sugar
Call 1-800-645-2158, 9am–5pm ET, Monday - Friday
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SEAL UNDER CAP IS BROKEN OR MISSING.
NDC 0536-3297-36
Children’s Aspirin (NSAID) 11/4 gr (81 mg)
Pain Reliever
Chewable Orange Flavor
36 Tablets
Rugby Duluth, Georgia 30097
|
ASPIRIN
aspirin tablet | ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part343 | 01/23/1998 | |
| Labeler - Rugby Laboratories Inc. (191427277) |
| Registrant - Advance Pharmaceutical Inc. (078301063) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Advance Pharmaceutical Inc. | 078301063 | MANUFACTURE | |