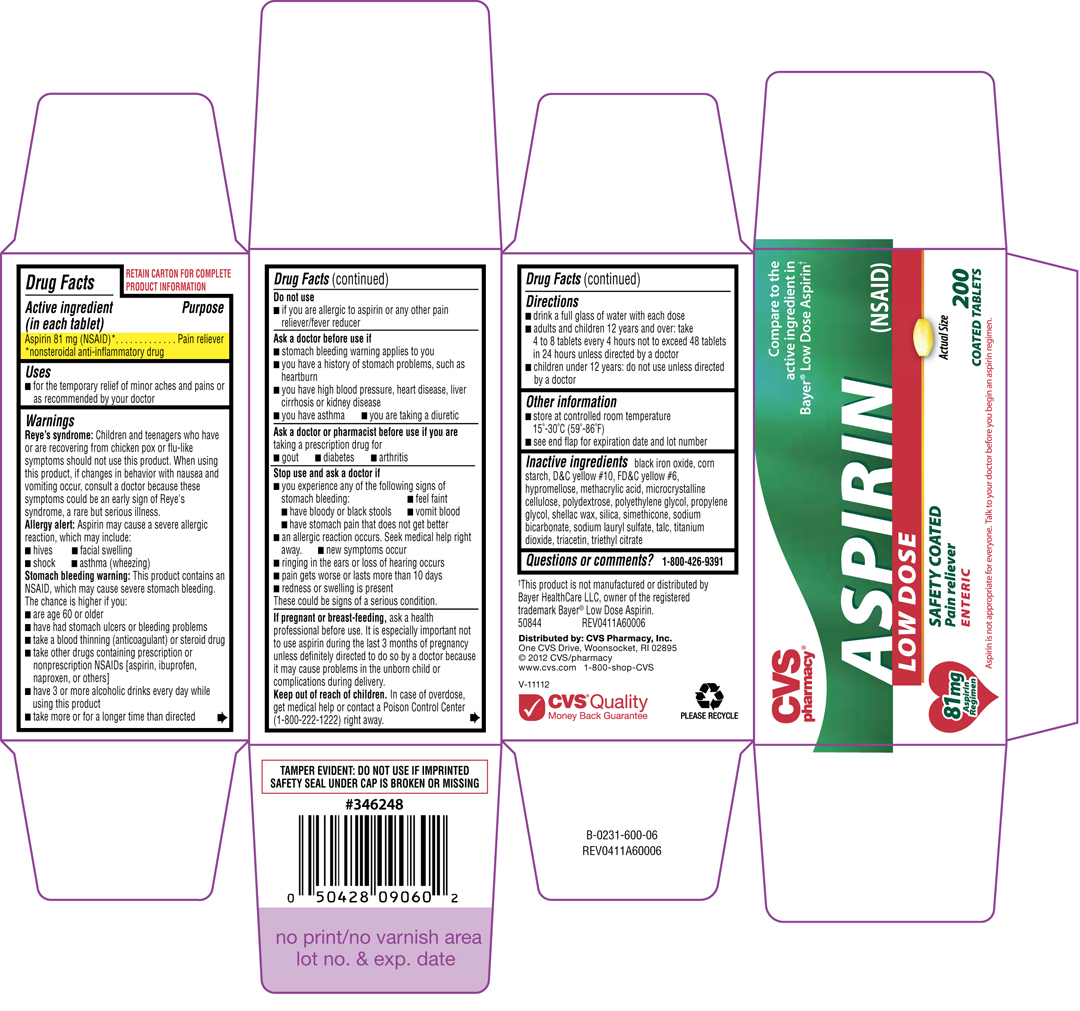
ENTERIC COATED ASPIRIN
-
aspirin tablet
WOONSOCKET PRESCRIPTION CENTER,INCORPORATED
Aspirin 81 mg (NSAID)*
*nonsteroidal anti-inflammatory drug
Pain reliever
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction, which may include:
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
taking a prescription drug for
These could be signs of a serious condition.
ask a health professional before use. It is especially important not to use aspirin during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
In case of overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
black iron oxide, corn starch, D&C yellow #10, FD&C yellow #6, hypromellose, mtehacrylic acid, microcrystalline cellulose, polydextrose, polyethylene glycol, propylene glycol, shellac wax, silca, simethicone, sodium bicarbonate, sodium lauryl sulfate, talc, titanium dioxide, triacetin, triethyl citrate
1-800-426-9391
CVS
pharmacy®
Compare to the active ingredient in Bayer® Low Dose Aspirin†
ASPIRIN (NSAID)
LOW DOSE
SAFETY COATED
Pain reliever
ENTERIC
200
COATED TABLETS
81mg Aspirin Regimen
Aspirin is not appropriate for everyone. Talk to your doctor before you begin an aspirin regimen.
50844 REV0411A60006
Distributed by: CVS Pharmacy, Inc.
One CVS Drive, Woonsocket, RI 02895
©2011 CVS/pharmacy
www.cvs.com 1-800-shop-CVS
V-11112
CVS 44-600
|
ENTERIC COATED ASPIRIN
aspirin tablet | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC MONOGRAPH NOT FINAL | part343 | 02/12/2022 | |
| Labeler - WOONSOCKET PRESCRIPTION CENTER,INCORPORATED (062312574) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 832867894 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 038154464 | PACK | |