LOW DOSE ASPIRIN
-
aspirin tablet
L.N.K. International, Inc.
Aspirin 81 mg (NSAID)*
*nonsteroidal anti-inflammatory drug
Pain reliever
Reye's syndrome: Children and tennagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction, which may include:
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
taking a prescription drug for
These could be signs of a serious condition.
ask a health professional before use. It is especially important not to use aspirin during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
In case of overdose, get medical help or contact a Poison Control Center right away.
black iron oxide, corn starch, D&C yellow #10, FD&C yellow #6, hypromellose, methacrylic acid, microcrystalline cellulose, ploydextrose, polyethylene glycol, propylene glycol, shellac wax, silica, simethicone, sodium bicarbonate, sodium lauryl sulfate, talc, titanium dioxide, tracetin, triethyl citrate
1-800-426-9391
Quality Plus
NDC 50844-563-32
†Compare to the active ingredient in Bayer® Low Dose Aspirin
LOW DOSE
Aspirin 81 mg
Pain Reliever (NSAID) ASPIRIN REGIMEN
120 Enteric Coated Tablets Safety Coated
†This product is not manufactured or distributed by Bayer HealthCare LLC, owner of the registered trademark Bayer® Low Dose Aspirin.
50844 ORG041160032
Distributed by LNK INTERNATIONAL, INC.
60 Arkay Drive, Hauppauge, NY 11788
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
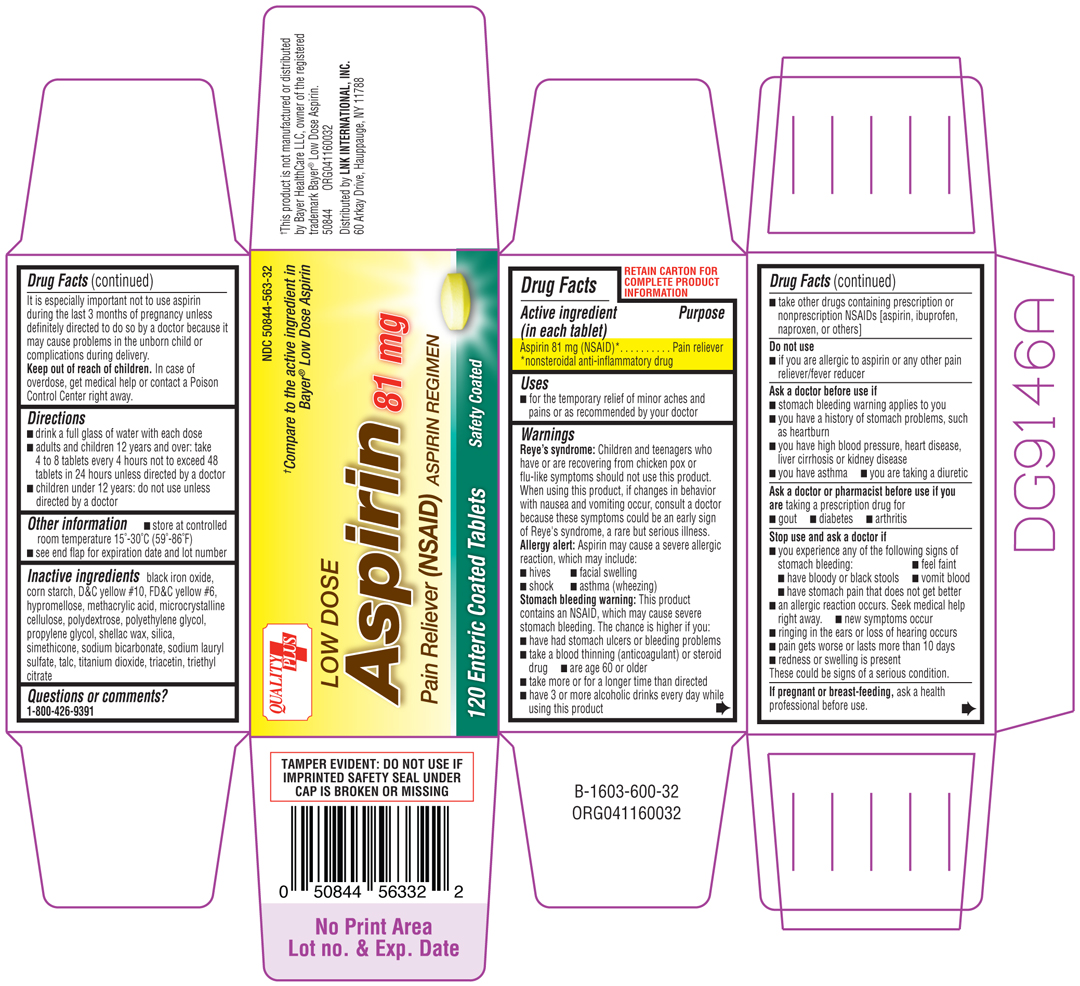
B-1603-600-32 ORG0411
|
LOW DOSE ASPIRIN
aspirin tablet | ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC MONOGRAPH FINAL | part343 | 05/02/2023 | |
| Labeler - L.N.K. International, Inc. (038154464) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 832867894 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| LNK International, Inc. | 038154464 | PACK | |