ASPIRIN
-
aspirin tablet, chewable
Publix Super Markets Inc
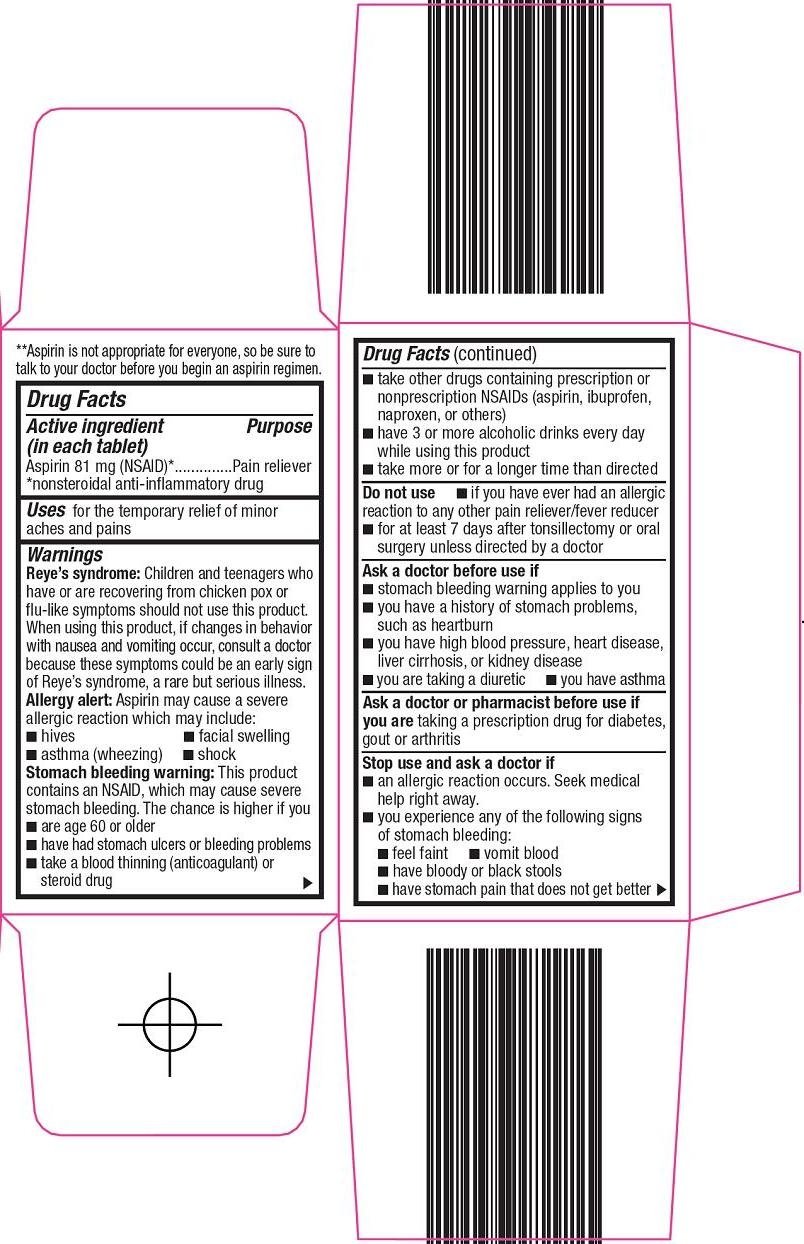
Aspirin 81 mg (NSAID)*
*nonsteroidal anti-inflammatory drug
Pain reliever
for the temporary relief of minor aches and pains
Reye’s syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye’s syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction which may include:
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you
taking a prescription drug for diabetes, gout or arthritis
These could be signs of a serious condition
ask a health professional before use. It is especially important not to use aspirin during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
corn starch, D&C red #27 aluminum lake, dextrose excipient, FD&C red #40 aluminum lake, flavors, saccharin sodium
LOW DOSE
chewableaspirin
ASPIRIN REGIMEN
81 mg
PAIN RELIEVER (NSAID)
cherry flavor
ACTUAL SIZE
81 mg each
Compare to active ingredient in Bayer® Chewable Aspirin

Chewable Aspirin Carton Image 1
Chewable Aspirin Carton Image 2
|
ASPIRIN
aspirin tablet, chewable | ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part343 | 08/24/1998 | |
| Labeler - Publix Super Markets Inc (006922009) |