ASPIRIN
-
aspirin tablet, delayed release
TIME CAP LABORATORIES INC.
Each tablet contains - Aspirin 325 mg (NSAID)
Keep Out of the Reach of Children: In case of overdose, get medical help or contact a Poison Control Center right away
PAIN RELIEVER
For the temporary relief of minor aches and pains due to: muscle pain, toothache, headache, colds, menstrual pain, minor pain of arthritis or as directed by your physician
Drink a full glass of water with each dose. Adults and children 12 years and over; take 1 to 2 tablets every 4 hours while symptoms last. Do not take more than 12 tablets in 24 hours unless directed by a doctor. Children under 12 years; consult a doctor.
Reye’s syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye’s syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction, which may include: facial swelling, shock, hives, asthma (wheezing)
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you are age 60 or older, have had stomach ulcers or bleeding problems, take a blood thinning (anticoagulant) or steroid drug, take other drugs containing prescription or nonprescription NSAIDS(aspirin, ibuprofen, naproxen or others), have 3 or more alcoholic drinks every day while using this product, take more or for a longer time than directed.
acetylted monoglycerides, corn starch, croscarmellose sodium,D-C yellow #10, FD-C yellow #6, hypromellose,hypromellose phthalate,microcrystalline cellulose,mineral oil,polyethylene glycol (PEG) 400, polysorbate 80, titanium dioxide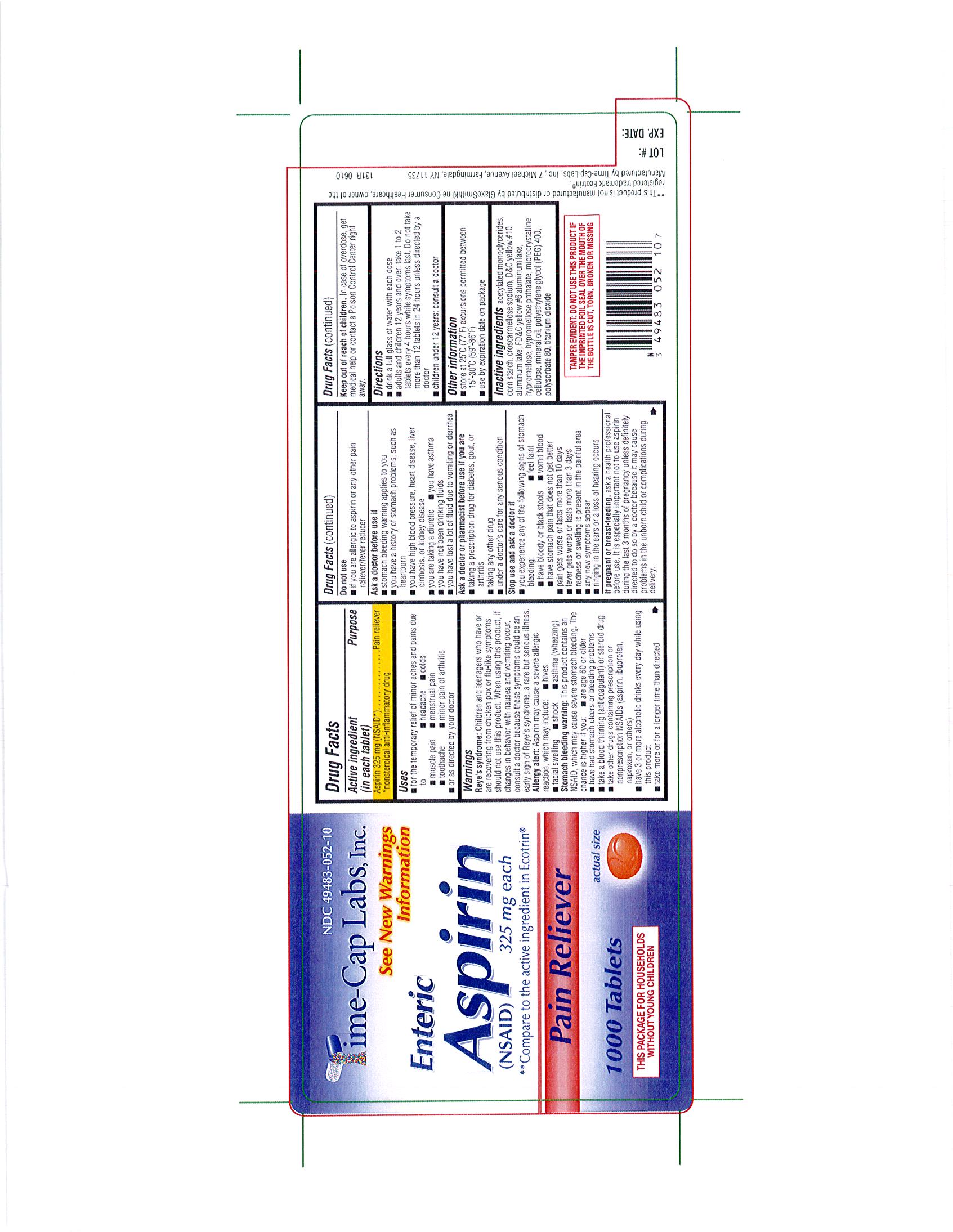
|
ASPIRIN
aspirin tablet, delayed release | ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part343 | 05/08/2022 | |
| Labeler - TIME CAP LABORATORIES INC. (037052099) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| TIME CAP LABORATORIES, INC. | 037052099 | manufacture | |