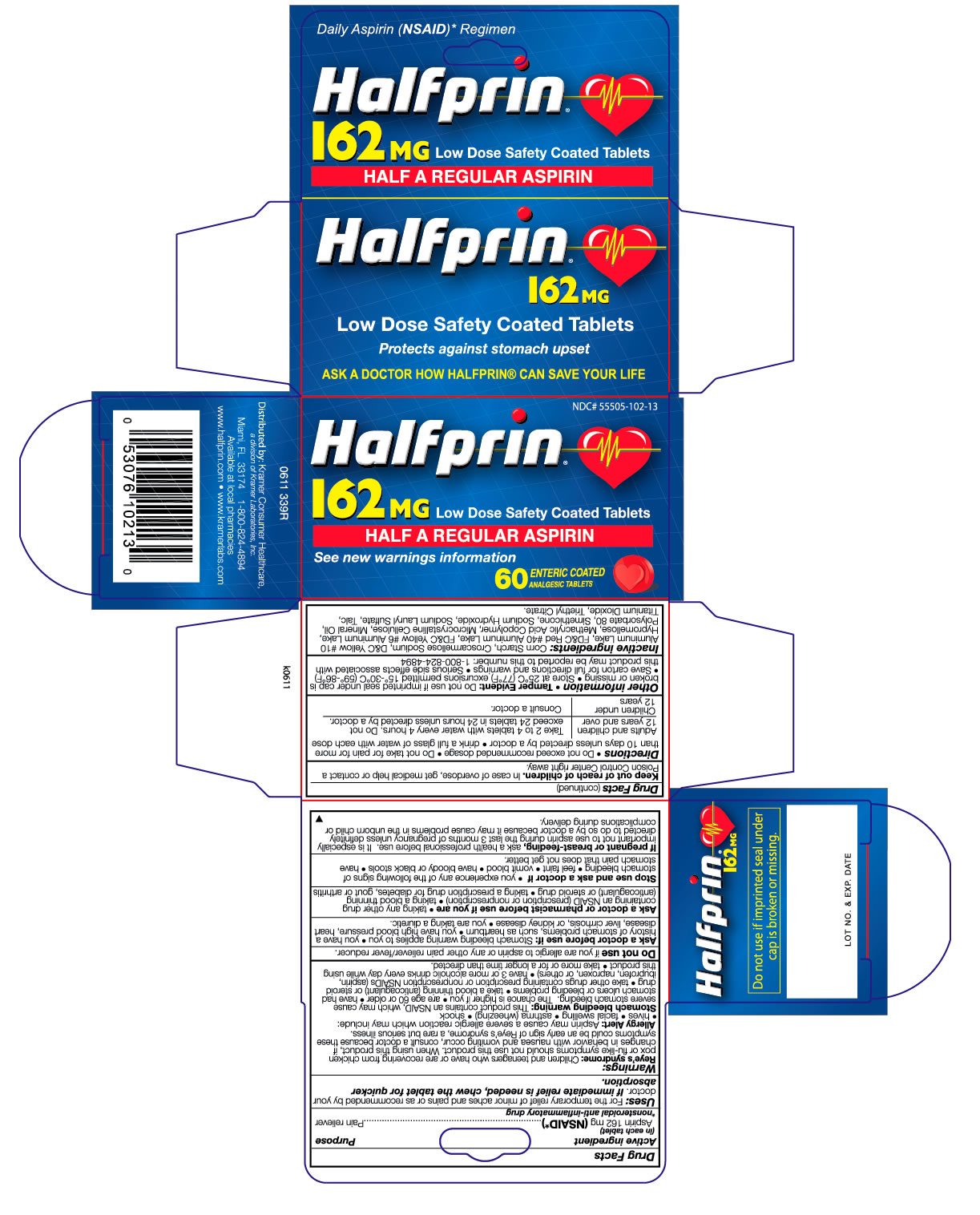
HALFPRIN LOW DOSE ASPIRIN
-
aspirin tablet, coated
Kramer Laboratories
Aspirin 162 mg
Pain reliever
Keep out of reach of children. In case of overdose, get medical help or contact a
Poison Control Center right away.
For the temporary relief of minor aches and
pains or as recommended by your doctor. If
immediate relief is needed, chew the tablet
for quicker absorption.
Directions: drink a full glass of water with each dose; adults and children 12 years and over: take 2 to 4 tablets every 4 hours not to exceed 24 tablets in 24 hours unless directed by a doctor
Children under 12 years - consult a doctor
Reye’s syndrome: Children and teenagers who have or are recovering from chicken pox
or flu-like symptoms should not use this product. When using this product, if changes
in behavior with nausea and vomiting occur, consult a doctor because these symptoms
could be an early sign of Reye’s syndrome, a rare but serious illness.
Allergy Alert: Aspirin may cause a severe allergic reaction which may include:
• hives • facial swelling • asthma (wheezing) • shock
Stomach bleeding warning: This product contains an NSAID, which may cause
severe stomach bleeding. The chance is higher if you • are age 60 or older • have had
stomach ulcers or bleeding problems • take a blood thinning (anticoagulant) or steroid
drug • take other drugs containing prescription or nonprescription NSAIDs (aspirin,
ibuprofen, naproxen, or others) • have 3 or more alcoholic drinks every day while using
this product • take more or for a longer time than directed.
 Enter section text here
Enter section text here
|
HALFPRIN
LOW DOSE ASPIRIN aspirin tablet, coated | ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part343 | 08/31/2011 | |
| Labeler - Kramer Laboratories (122720675) |
| Establishment | |||
| Name | Address | ID/FEI | Operations |
| Time Caps Labs | 037052099 | manufacture | |