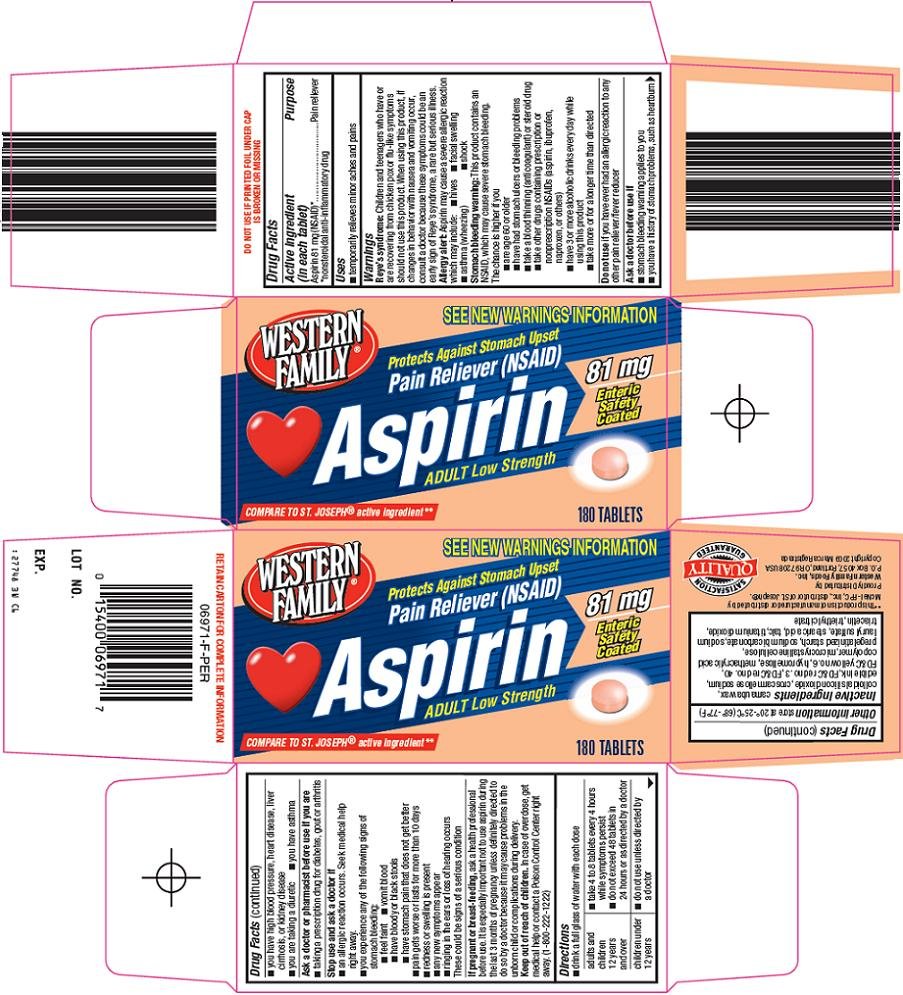
ASPIRIN ADULT LOW STRENGTH
-
aspirin tablet, film coated
Western Family Foods Inc
Aspirin 81 mg (NSAID)*
*nonsteroidal anti-inflammatory drug
Pain reliever
Reye’s syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye’s syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction which may include:
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding.
The chance is higher if you
if you have ever had an allergic reaction to any other pain reliever/fever reducer
These could be signs of a serious condition
ask a health professional before use. It is especially important not to use aspirin during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
| adults and children 12 years and over |
|
| children under 12 years |
|
store at 20º-25ºC (68º-77ºF)
carnauba wax, colloidal silicon dioxide, croscarmellose sodium, edible ink, FD&C red no. 3, FD&C red no. 40, FD&C yellow no. 6, hypromellose, methacrylic acid copolymer, microcrystalline cellulose, pregelatinized starch, sodium bicarbonate, sodium lauryl sulfate, stearic acid, talc, titanium dioxide, triacetin, triethyl citrate
See New Warnings Information
Protects Against Stomach Upset
Pain Reliever (NSAID)
Aspirin
Adult Low Strength
81 mg
Enteric Safety Coated
Compare to St.Joseph® active ingredient
Aspirin 81 mg Carton
|
ASPIRIN
ADULT LOW STRENGTH aspirin tablet, film coated | ||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part343 | 05/19/2004 | |
| Labeler - Western Family Foods Inc (192166072) |