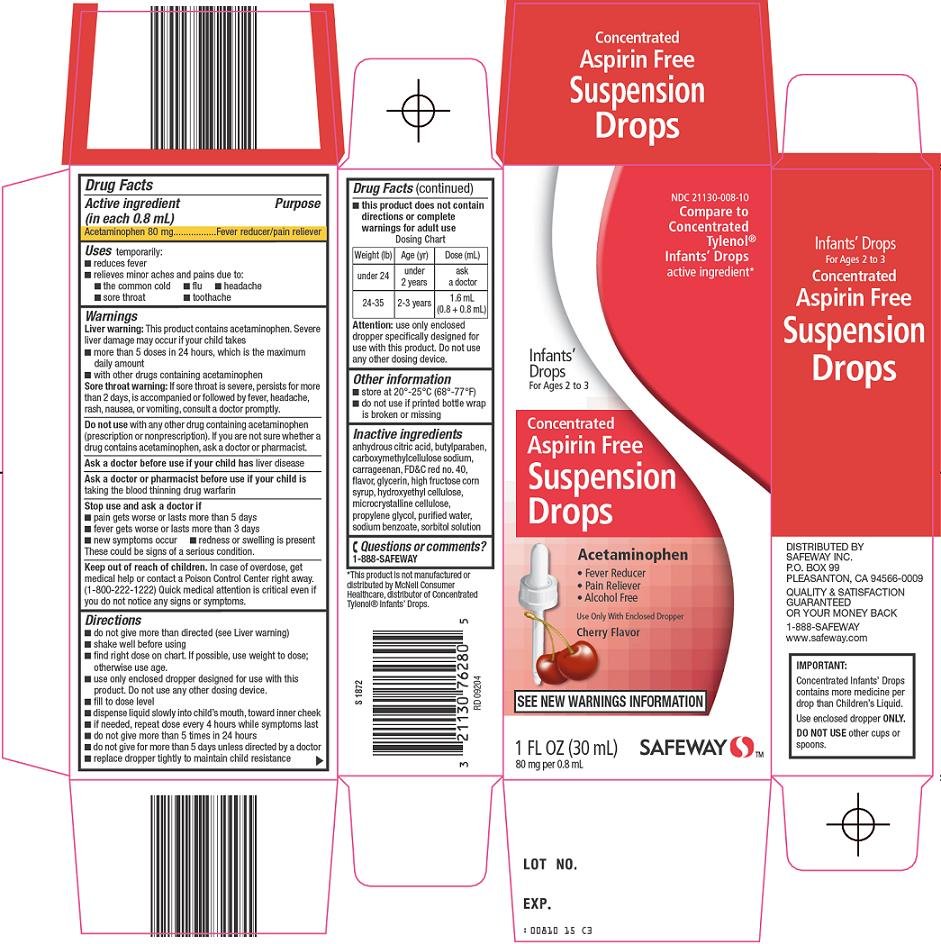
INFANTS CONCENTRATED ASPIRIN FREE
-
acetaminophen suspension/ drops
Safeway
Acetaminophen 80 mg
Fever reducer/pain reliever
temporarily:
Liver warning: This product contains acetaminophen. Severe liver damage may occur if your child takes
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
liver disease
taking the blood thinning drug warfarin
These could be signs of a serious condition.
In case of overdose, get medical help or contact a Poison Control Center right away. (1-800-222-1222) Quick medical attention is critical even if you do not notice any signs or symptoms.
| Dosing Chart | ||
| Weight(lb) | Age(yr) | Dose (mL) |
| under 24 | under 2 years | ask a doctor |
| 24-35 | 2–3 years | 1.6 mL (0.8 + 0.8 mL) |
Attention: use only enclosed dropper specifically designed for use with this product. Do not use any other dosing device.
anhydrous citric acid, butylparaben, carboxymethylcellulose sodium, carrageenan, FD&C red no. 40, flavor, glycerin, high fructose corn syrup, hydroxyethyl cellulose, microcrystalline cellulose, propylene glycol, purified water, sodium benzoate, sorbitol solution
1-888-SAFEWAY
Compare to Concentrated Tylenol® Infants’ Drops active ingredient
Infants’ Drops
For Ages 2 to 3
Concentrated
Aspirin Free
Suspension Drops
Acetaminophen
Fever Reducer
Pain Reliever
Alcohol Free
Use Only with Enclosed Dropper
Cherry Flavor
See New Warnings Information
80 mg per 0.8 mL
Concentrated Suspension Drops Carton
|
INFANTS
CONCENTRATED ASPIRIN FREE acetaminophen suspension/ drops | ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| ||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph not final | part343 | 09/17/1997 | |
| Labeler - Safeway (009137209) |