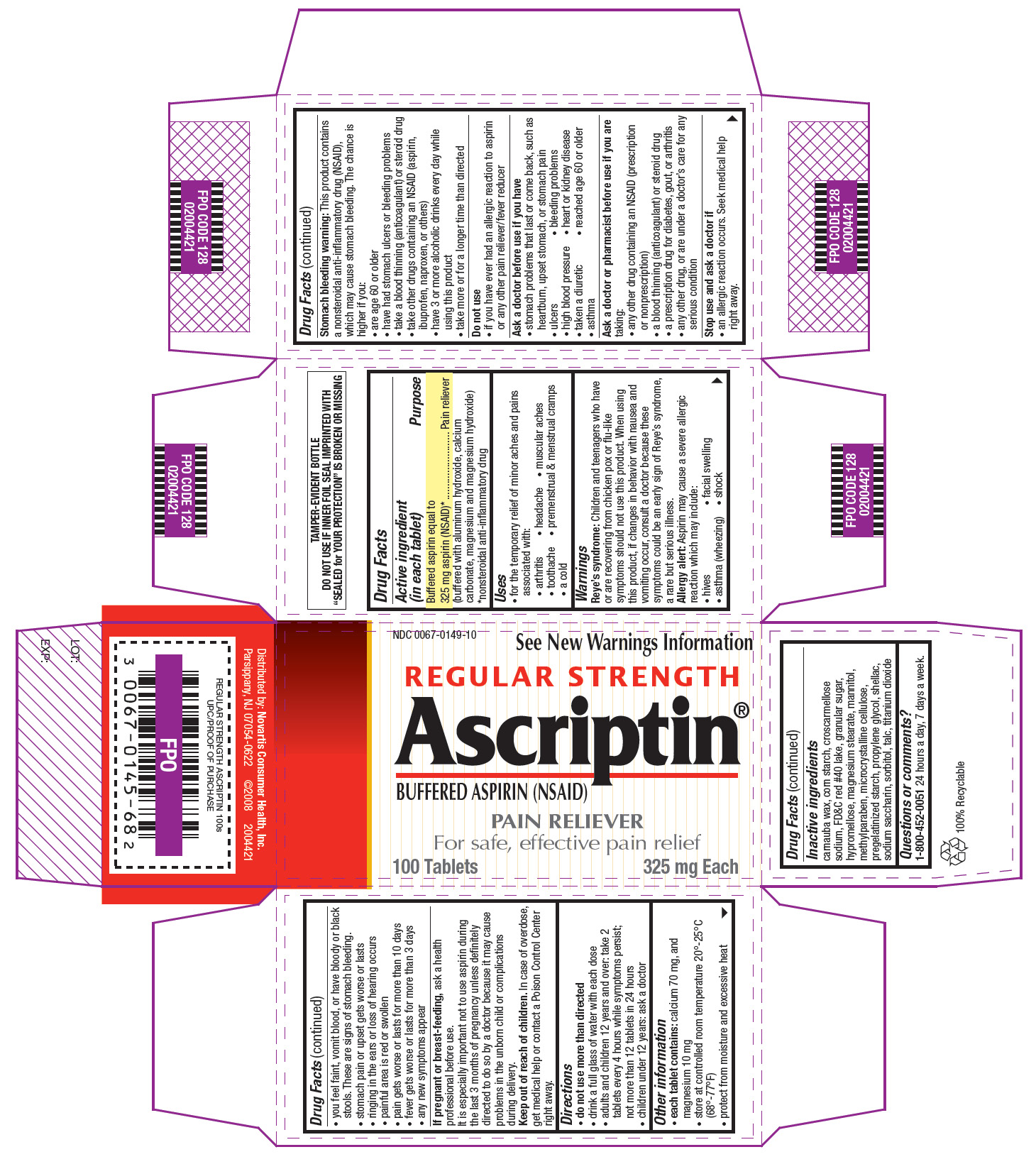
ASCRIPTIN REGULAR STRENGTH BUFFERED ASPIRIN
-
aspirin tablet, film coated
Novartis Consumer Health, Inc.
Buffered aspirin equal to 325 mg aspirin (NSAID)*
(buffered with aluminum hydroxide, calcium carbonate, and magnesium hydroxide)
*nonsteroidal anti-inflammatory drug
Pain reliever
Reye’s syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye’s syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction which may include
Stomach bleeding warning: This product contains a nonsteroidal anti-inflammatory drug (NSAID), which may cause stomach bleeding. The chance is higher if you:
If pregnant or breast-feeding, ask a health professional before use.
It is especially important not to use aspirin during the last 3 months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Inactive ingredients carnauba wax, corn starch, croscarmellose sodium, FD&C red #40 lake, granular sugar, hypromellose, magnesium stearate, mannitol, methylparaben, microcrystalline cellulose, pregelatinized starch, propylene glycol, shellac, sodium saccharin, sorbitol, talc, titanium dioxide
1-800-452-0051
Novartis Consumer Health, Inc.
200 Kimball Drive
Parsippany, NJ 07054-0622
Ascriptin
|
ASCRIPTIN
REGULAR STRENGTH BUFFERED ASPIRIN aspirin tablet, film coated | ||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||
| Marketing Information | |||
| Marketing Category | Application Number or Monograph Citation | Marketing Start Date | Marketing End Date |
| OTC monograph final | part343 | 09/24/2009 | |
| Labeler - Novartis Consumer Health, Inc. (879821635) |